j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
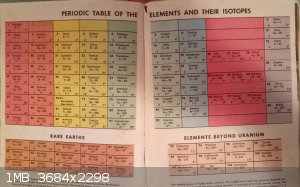
Unusual periodic table
Over Christmas I was browsing the bookshelves at the house where I grew up and I came across the first periodic table that I ever saw. The book was
part of a Disney series dated around 1970 (I forgot to check).
Anyway, the layout is unusual -- I think obsolete even in that day. Also interesting to note no confirmed name for nobelium. (Flerovium and
joliotium were other names floating around along with some uncorroborated and later retracted data from Swedish experimentation.)
I can see some logic to this arrangement. But it is strange to see Cu, Ag and Au among the group I metals.
Has anyone else seen this arrangement before? What about other historical arrangements?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Oh, yes, that's the abbreviated version that's been floating around since shortly after Mendeleev wrote up the original, with the IA and IB metals
lumped together.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Ogannessionn
Harmless

Posts: 5
Registered: 19-12-2018
Location: Earth
Member Is Offline
Mood: Hydrophilic
|
|
So what happened to Moscovium, Tennesine and Oganesson.
  
|
|
|
wakatutu
Harmless

Posts: 31
Registered: 23-2-2017
Member Is Offline
Mood: Mostly Harmless
|
|
https://en.wikipedia.org/wiki/Alternative_periodic_tables
I'm rather fond of Benfey's snail model
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Hmm it cant be they werent discovered yet...
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Well, since you raise the matter, Ogannessionn, there's another quirk about this table, or at least the date when it was made. The heaviest element is
103 - right at the end of the actinides. Not explicit in this representation, but I imagine that there was a sense that the table was somehow
complete. A bit like now where we have reached the end of row 7. There is something kind of tidy and organised about having complete rows and no
space-fillers.
Or was that not how people viewed it in the early seventies?
I know that when I first began learning chem the 7th row was full of blanks and systematic names and disputed names and no two tables were the same.
It was like it was torn off at the bottom.
|
|
|
j_sum1
|
Thread Split
14-1-2019 at 15:21 |
j_sum1
|
Thread Pruned
16-1-2019 at 11:27 |