ChemistryForever
Hazard to Self
 
Posts: 64
Registered: 6-12-2018
Member Is Offline
|
|
Astatine probable vapor colour
What would be the expected color of an astatine vapor (supposing someone could synthesize it very fast until it decomposes ... )? ( example iodine-
violet ).
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Much about Astatine is unknown as it very quickly decomposes. The pure element has never been synthesized and if it was it would be vaporized by the
heat of its own radiation. If we could see it, I suspect it would be a dark brown or even black as the color of halogens gets darker as you go down.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Mr. Rogers
Hazard to Others
  
Posts: 184
Registered: 30-10-2017
Location: Ammonia Avenue
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ChemistryForever  | | What would be the expected color of an astatine vapor (supposing someone could synthesize it very fast until it decomposes ... )? ( example iodine-
violet ). |
If you go by Iodine and Bromine vapor, it's probably not a logical stretch to say it would be very dark brown. The halogens seem to be very
consistent like this.
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
Would it be possible to calculate the approximate colour by working from the molecular orbitals? These have been calculated, and the colour will come
from electronic transitions within a molecule of At2. Being a gas simplifies the problem enormously.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Quote: Originally posted by 12thealchemist  | | Would it be possible to calculate the approximate colour by working from the molecular orbitals? These have been calculated, and the colour will come
from electronic transitions within a molecule of At2. Being a gas simplifies the problem enormously. |
Exactly my thoughts. This problem should be exceedingly simple to solve computationally; unfortunately I don't have Gaussian at the moment (back home
for Christmas) but an accurate calculation of MOs should give the absorbed colour with a great accuracy.
It's not as if anyone is going to try this empirically... especially in a backyard shed!
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I can confidently assert that it is green and yellow in diagonal stripes.
Let me know when anyone actually proves that I'm wrong.
On a related note, does anyone have any software that can predict, from first principles, that iodine vapour (near atmospheric pressure and , say,
100C) is red?
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mr. Rogers  |
If you go by Iodine and Bromine vapor, it's probably not a logical stretch to say it would be very dark brown. The halogens seem to be very
consistent like this. |
If you look at iodine and bromine vapours it's clear that they are very different- one is brown , one is red.
Hardly "consistent"
|
|
|
DraconicAcid
International Hazard
    
Posts: 4413
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Iodine vapour is brown? It's always looked beautifully purple to me.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Tsjerk
International Hazard
    
Posts: 3035
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by unionised  | I can confidently assert that it is green and yellow in diagonal stripes.
Let me know when anyone actually proves that I'm wrong.
On a related note, does anyone have any software that can predict, from first principles, that iodine vapour (near atmospheric pressure and , say,
100C) is red? |
How did you come up with these colors?
I don't know the color of a somewhat pure vapor, but I'm pretty sure the surroundings will be blue colored because of the Cherenkov radiation.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
The results of your first principle calculations will depend on whether the vapours are monoatomic or diatomic, which I understand is presently
unknown.
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
To me, bromine vapour is reddish brown, more red than nitrogen dioxide but less red than chromyl chloride vapour. Iodine vapour is unequivocally
purple. I would assume that astatine behaves like the rest of the halogens in forming dimers, although the calculation could in theory be run for a
monomeric vapour too.
|
|
|
Mr. Rogers
Hazard to Others
  
Posts: 184
Registered: 30-10-2017
Location: Ammonia Avenue
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by Mr. Rogers  |
If you go by Iodine and Bromine vapor, it's probably not a logical stretch to say it would be very dark brown. The halogens seem to be very
consistent like this. |
If you look at iodine and bromine vapours it's clear that they are very different- one is brown , one is red.
Hardly "consistent" |
I don't mean they're the same color - I mean the properties of this group have been very predictable.
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
There is this diagram on Wikipedia on the Tennessine page:
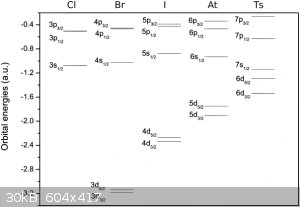
|
|
|