RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Water desalination in deep sea water - using pressure of the deep - possible?
So the way a lot of water desalination works is through filters like RO membrane filters where water is pushed through the membranes by a high
pressure differential across the membrane. IDK how much pressure is needed, let's just say 1000psi which would be equal to about 2200ft depth(1000psi
= 66 ATM; 1 ATM = 33ft = 33*66 = ~1000ft deep).
So if a bunch of filters were placed at this depth, or deeper, and a pipe was run to the surface, then as water was pumped out of the filter center
(into the pipe) the pressure of the water would force the salt water through the membrane.
Now I see that this shouldn't work because of the weight/pressure of the column of water in the pipe running to the surface, as this creates back
pressure creating resistance to the water flowing through the membrane.
I'm trying to figure out if there is a way to make this work, possibly by using filters with very large surface area and a small diameter pipe running
to the surface - much like how hydraulic pump work.
If the membrane surface was 100 ft^2 and the exit pipe was 1" diameter then the weight of the column of water is much lower than a larger pipe, but
still exerts the same PSI on the filter inside. But this is where I see other issues and think that this won't work at all.
Now if a large 100,000 gallon tank (filled with air) was lowered to 2233 ft (the extra 33ft to negate the 1ATM of air pressure inside the tank), and a
pipe was attached to a filter bank, then the water would flow through the filters and into the tank until it was full. It could then be lifted (with
inflatable air bags or crane??) to the surface and drained - repeat. IDK how pumping the water from the tank would work (as far as energy required),
possibly by having an air bladder in the tank which could be filled with air much like domestic air bladder tanks for homes with wells. Once the water
was removed, the pressure in the bladder would be released and the air would flow back out from where it was pumped and new filtered water would flow
back into the tank and the cycle could be repeated.
Now IDK if there would be any energy savings between pumping water through filters at the surface. The water (if being the same salinity at any
pumping location) requires the same pressure to pass through the filter, I'm just wondering if there is any benefit to using sea pressure to do this
and if it is easier to compress air into tanks which could be lowered to fill float bags to raise the tanks - or pumping air to push the water out of
the tanks. Or if a chemical reaction taking place in a container to release gas to float the tank or create pressure to fill the bladder.
Just wondering if there are any ways that this process could be used in VERY large amounts of water, like the amounts needed to service large cities.
I would think that there would be increasing benefits the larger the process goes, especially when talking about tank size & material used (being
volume is cubed).
|
|
|
weilawei
Hazard to Others
  
Posts: 130
Registered: 3-12-2017
Member Is Offline
Mood: No Mood
|
|
If you remove water from a pipe immersed in thing of water, the entire surrounding water will drop until the level is even again. You only have to
pull clean water out of the pipe. Treat the topside like a well--don't make a water tight seal from the pipe to the pump, so you aren't trying to
raise a column of water. Let the ocean do the heavy pumping forcing it up through your filter. Seems sane. I think.
I'm also very tired on a Friday. Who wants to poke holes in the idea?
I also threw together a 5 minue test rig. Piece of plastic pipe, bucket, water/oil/garbage. I taped a rag over the bottom of the pipe and taped a
ruler to it extending 1 inch past the bottom. I placed it in the bucket of water and siphoned water off the top (you'd need a real pump in the ocean,
but I can put my ocean up higher than the sink).
Worked fine. I imagine it would scale quite well aside from the usual bio difficulties of putting anything in the ocean.
  
[Edited on 3-8-2018 by weilawei]
|
|
|
streety
Hazard to Others
  
Posts: 110
Registered: 14-5-2018
Member Is Offline
|
|
The flow rate across the membrane is going to be proportional to the pressure difference across it. This means the important point is not how deep the
membrane is in the ocean but what is the difference in depth between the salt water and fresh water sides.
I haven't looked up details for a suitable membrane but you will either need a massive surface or a big difference in depth.
Overall, I don't think the depth of the ocean can be productively used for desalination. The tank idea is interesting but I suspect the work needed to
either lower or raise the tank would be much the same (with added inefficiencies) as that needed to pressurize water at the surface.
|
|
|
weilawei
Hazard to Others
  
Posts: 130
Registered: 3-12-2017
Member Is Offline
Mood: No Mood
|
|
If you pull water out the top, the height difference will increase until the pressure difference is great enough to force water across the filter, and
the height difference reaches equilibrium.
So, you need a pipe long enough to allow that height and pressure difference which imposes a minimum depth requirement for the body of water. As to
the pump at the top, you can use any flow rate which doesn't exceed the flow rate across the filter for a given height/pressure difference for
continuous operation--ideally equal so the level remains constant. Situate the whole rig on a buoy to account for tides. You could literally also
come by with a bucket and do the well thing.
On further thought, the buoy and pipe setup, being buoyant, would have issues. Anchored to the bottom would work better. Perhaps a system of buoyancy
compensation could be used for floating rigs.
[Edited on 3-8-2018 by weilawei]
|
|
|
streety
Hazard to Others
  
Posts: 110
Registered: 14-5-2018
Member Is Offline
|
|
You need energy to raise the water the thousand feet+ you need for the process to produce any amount of fresh water. That is a thousand feet the level
of the fresh water needs to be below the level of the seawater.
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
The more I looked at it, there doesn't seem to be any way that it passes through the membrane and reach the surface w/o pumping basically the same
pressure needed to pass through the membrane.
If you lower (to 2200ft or 1000psi) a large filter, say the size of a 55 gallon drum, with a 3" pipe attached to it, the drum would fill with water
but once the drum/filter is filled I think it would stop allowing more water in due to the pressure differential. If you had 33ft (1 ATM or 14.7psi)
of water in the 3" pipe, that would drop the pressure differential to 985.3 psi which wouldn't be enough for the water to pass through the membrane
(b/c it needs 1000psi to pass though - or there abouts).
Now if you applied vacuum to the pipe, you can only draw water up 1ATM worth of water, or 33ft, and then no more water would flow in.
Now if the the filter/barrel & 3" pipe were lowered 2x as deep, the water would fill the tank completely and the 3" tube 1/2 way where the
pressure of the water in the 3" pipe (weight of water in the column) plus the pressure needed to pass through the membrane is equal to the outside
pressure of the water.
I think what would be needed is to have something like a barrel/filter submerged to the depth needed to get the PSI to pass through the filter and
then have an extraction pipe running through an air supply/pressure equaliztion pipe to the surface. The water would have to be pumped up 2200ft but
with this method there wouldn't be a need to pump water through a membrane. IDK if it takes more energy to pump water up that distance or to create
the pressure needed to pass through the membrane, it seems like it would be about the same being that they both equal 1000psi.
|
|
|
weilawei
Hazard to Others
  
Posts: 130
Registered: 3-12-2017
Member Is Offline
Mood: No Mood
|
|
It seems like you would pay a similar price either way. It's a shame. It looked promising, but you're right. You'd have to lift a column of water the
same volume as whatever you want to draw off (after the initial pumping out of the pipe) to a ~700m height. That's going to take as much power as
forcing the water through a filter at the same pressure (a bit north of 6895 kilonewtons/m^2) and flow rate..
Even living down there wouldn't help you. You'd let the water into your habitat for free, but then you'd still require the same energy to pump it back
out after you've run it through your various bits of biology. Stand pipe going back up? Back to the water lifting issue.
TANSTAAFL.
[Edited on 4-8-2018 by weilawei]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Whenever you think you can extract energy from something, you need to ask yourself: is this a perpetual motion machine?
If you could bring pressurized ocean water to the surface without compromising on the pressure, or spending an equivalent amount of energy, you could
then use that pressure to make energy, release the water at surface level, and go on extracting energy like this forever -- and that should be
impossible! It's a perpetual motion machine. Therefore, it won't work.
|
|
|
streety
Hazard to Others
  
Posts: 110
Registered: 14-5-2018
Member Is Offline
|
|
Completing the circle, an approach for extracting energy from mixing seawater and fresh water: http://www.bbc.com/future/story/20150610-blue-energy-how-mix...
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
You need an energy source. Pressure is not an energy source. A pressure diffetential is.
Worthwhile remembering is the Bernoulli formula. You can calculate the energy state of a fluid: there are three terms. There is gravitational
potential energy associated with the height of the water and its density. There is energy associated with the pressure. And there is kinetic ednergy
if the fluid is moving.
In any given body of water the energy is constant throughout. Near the top there is more gravitational energy and less pressure. Deep down there is
more pressure and less gravitational energy.
Drill a hole in a bucket and you will have a jet of water come out. The jet has kinetic energy but no pressure as there is nothing to constrain it.
Energy will leave the system as water leaves the bucket. This energy can be harnessed.
You don't have the same opportunity to extract energy from an ocean body even if the pressure is high. You cannot set up a pressure differential and
so you cannot induce a flow and hence cannot get harnassable kinetic energy.
|
|
|
MJ101
Hazard to Self
 
Posts: 82
Registered: 14-6-2018
Member Is Offline
Mood: Always Sunny
|
|
@j_sum1: That same Bernoulli formula comes into play when considering the aquatic life around the water inlet.
If the inlet flow is too fast, the fish will get sucked into the intake, messing up the ecosystem and making double work.
https://www.youtube.com/watch?v=VSEbpqQyegY
I don't know much about the desalinization plant in Melbourne, aside from what is shown in this video, but it looks amazing.
[Edited on 4-8-2018 by MJ101]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Yes it takes almost as much energy to get the desalinated water back to the surface as you save not having to pressurise the salty water. As one
poster said “no perpetual motion machines“.
However the density of sea water is about 3% greater than fresh water. So if you place the filter at the deepest point of all the oceans, about
33,000ft the difference in density between the salty water column and the fresh water column would produce a differential of about 1000psi and
therefore the fresh water would flow out of the 33,000ft high return pipe at the surface. I am not suggesting such a system is practical or feasible
though. The higher salinity and low temperature of deep sea water may make it even more impractical or impossible.
As already stated there are no perpetually motion machines so where does the energy comes from to drive such a system? I guess it’s the
gravitational energy of having the higher density more salty water at the bottom of the ocean.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48  |
However the density of sea water is about 3% greater than fresh water. So if you place the filter at the deepest point of all the oceans, about
33,000ft the difference in density between the salty water column and the fresh water column would produce a differential of about 1000psi and
therefore the fresh water would flow out of the 33,000ft high return pipe at the surface. I am not suggesting such a system is practical or feasible
though. The higher salinity and low temperature of deep sea water may make it even more impractical or impossible.
|
I think you have forgotten to take account of osmotic pressure across the membrane.
It's about 27 bar.
There's certainly something wrong, or you would have a perpetual motion machine.
Fundamentally, desalination needs energy.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Taking seawater to be 1030 kg/m^3, the pressure difference at a depth of 10 km (for reference, the deepest point in the ocean is 10.9 km) would be:
10000 m * 30 kg/m^3 * g = 2,940,000 Pa = 2.94 MPa
The osmotic pressure of seawater relative to fresh water is approximately 2.8 MPa.
So it looks like the idea is barely possible in theory. Unionised, to answer your point about where the energy would come from, it
would come from lowering the salt down the Earth's gravitational field.
In practice, there would need to be some additional pressure gradient (since an ~0.1 MPa difference isn't large enough to drive the water through at a
fast enough rate), which could be provided by removing water from the fresh water column. Of course, I don't think this strategy would be very
commercially viable.
EDIT: Consider the classic "U-shaped tube" experiment in osmosis. This would be pretty much the same experiment, although on a much larger scale.
[Edited on 8-4-2018 by Metacelsus]
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Metacelsus  | Taking seawater to be 1030 kg/m^3, the pressure difference at a depth of 10 km (for reference, the deepest point in the ocean is 10.9 km) would be:
10000 m * 30 kg/m^3 * g = 2,940,000 Pa = 2.94 MPa
The osmotic pressure of seawater relative to fresh water is approximately 2.8 MPa.
|
30 Kg/m^3? Surely that is wrong. Id calculate it like this:
1025 kg/m^3 * g / 1 m^2 * x = 10065.5 Pa/m * x = 2.8 * 10^6 Pa , which means:
x = 278.178 m and then add some to that for speed and subtract some for increased density due to lower temperature at lower depths and slight
compression of water...
Which makes it look plausible. Id imagine lifting water is more efficient than most compressors and accompanying pumps to move water around the plant.
[Edited on 4-8-2018 by Σldritch]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
The pressure comes from the density difference between seawater (1025 or 1030 kg/m^3) and fresh water (1000 kg/m^3). I assume the
interior of the tube is filled with fresh water, not air. This is where the 30 kg/m^3 figure comes from.
Of course, if the tube interior is filled with air then your calculation would make sense.
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Just thought I would add that at 4000m water actually compresses by 1.8%, IDK if that is a linear formula, so at 10.9km, it would be the same ratio or
not,I tend to think not though. Either way if it was linear than we would be looking at near 4.905% compression which would make the water a fair bit
denser in addition to the higher salinity. I suspect the compression may be higher, though I could be completely wrong and it could be near the same.
Anyone have any ideas on this?
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
I feel like this sort of idea might be practical if there was a saltwater dam somewhere to accomodate it. Then again, you could just use the energy
harnessed from any dam via conventional methods to run your high-pressure pump.
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Metacelsus  |
The pressure comes from the density difference between seawater (1025 or 1030 kg/m^3) and fresh water (1000 kg/m^3). I assume the
interior of the tube is filled with fresh water, not air. This is where the 30 kg/m^3 figure comes from.
Of course, if the tube interior is filled with air then your calculation would make sense. |
That makes sense though i see no good reason to fill the pipe with freshwater. A round pipe that can resist the pressure should be very easy to
construct commercially.
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
Only consideration that might work in your favor is dissolved gases in the water effervescing as it rises and the pressure drops providing a lifting
action to the water column. Lookup 'lake overturn' for the principle I'm referring to, providing these gases would pass through the filter.
Otherwise, pumping action can be provided using turbines in tidal currents.
The cost of lifting the column and replacing the filters would also be a major consideration.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
As wind, tidal flows and sunlight can all produce electricity, I would recommend an electrochemical approach with inexpensive carbon based electrodes.
Here is such an approach aimed at least reducing the chloride content of the sea water.
Construct a divided cell (composed of a salt bridge with membrane) where the water source around the electrode generating chlorine (designated as the
starting solution) is separated off. As Cl2 is not very soluble in water and even less so in water contaminated with chloride, the chlorine largely
escapes (perhaps harvested), thereby leaving the chloride concentration around that divided cell's electrode reduced.
The reduced chloride water is the starting solution in yet another divided cell setup,..... My suggested path is referred to as an electrodialysis
process (see https://www.amtaorg.com/electrodialysis-reversal-desalinatio... and https://www.wateronline.com/doc/ed-vs-ro-the-benefits-of-ele...) which employs electricity to draw salt ions out of solution through a membrane.
Here the cathode reaction also produces H2 which can be collected or burned as an energy source resulting in H2O.
Result is possibly a low chloride more alkaline water and having been treated with chlorine, also likely safe to drink as well.
General reference, see http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch20/fa...
Negatives are the expense of constructing the electric power component (wind turbines,..), cost of materials for creating/operating the consecutive
divided cells, cost of electrodes, membranes,....
[Edited on 16-8-2018 by AJKOER]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The force required to hold an empty pipe in the ocean at any depth would be enormous, due to the buoyancy of the air inside. Many of the
assumptions are looking an initial system, like an empty pipe. If the fresh water could fill the pipe, you would still have to pump it out, and once
there was water in the pipe, most of the pressure gradient would go away. These are interesting ideas, but you have to find a way to make them work
at equilibrium, which is the way that thermodynamic always wins over free energy systems. Even tidal water pressure generators, which on paper look
great, don't work very well in real life, as in the real world, you have friction, corrosion, slow equilibrium of water levels, waves, and more, which
often ablate most of the useful energy into heat.
This is no different from the fact that rain provides water at a height above sea level, which can provide "free" power. But there are many limits
on the practical use of hydroelectric power, many of which are political, aesthetic, environmental, legal, and practical, more than engineering
issues. That is why I laugh at so many ideas for "free" power, as even the real sources like hydroelectric, solar, and wind are all still quite
expensive in real life, due to costs of capital, equipment, labor, regulations, taxes, etc. Most people don't get that electricity costs about
$0.02/kwh to make (fuel cost), but it costs about 5 times that to get it to your house in a safe, reliable, consistent, legal, manner.
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
I use these Firestone airbags in industrial equipment. This is a suggested use by the manufacturer. With an array of these and some check valves you
may be able to generate enough power in situ to move the water column as necessary. Not perpetual motion, just tapping into solar/wind/tidal.
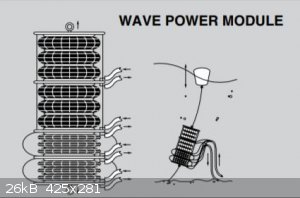
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I like this idea based on the dynamic power of tidals (see http://large.stanford.edu/courses/2010/ph240/chenw1/ ) to quote:
"In 1997, two Dutch engineers proposed a new method to utilize tidal energy, called Tidal Dynamic Power (TDP). [4,5] The method involves building a
large dam extended to the ocean and a long perpendicular barrier at its far end, forming a T-shape. Since the tidal current is parallel to the
coastlines near the coast, this system can accumulate large difference in water head on both sides of the dam twice per day. The difference of water
head can be used to generate, as in the barrage system. The estimated installed capacity of the dam can reach over 8 GW with less influence on the
ecosystem than barrage. Another benefit of this method is that it does not require a high natural tidal range, and can be applied in more sites than
other methods."
Adding nets to catch fish would provide jobs for transporting and sorting the fish. Profits could go to reduce operating cost of the TDP plant and for
electric power transmission.
[Edited on 27-8-2018 by AJKOER]
|
|
|