| Pages:
1
..
8
9
10
11
12
..
20 |
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
How many theoretical plates for that column? I'm guessing that you will have some great separations/purifications by distillation.
On another note: if you had everything, wouldn't you have to keep it everywhere? (Forgive me; it's been a long day  ) )
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by CharlieA  | How many theoretical plates for that column? I'm guessing that you will have some great separations/purifications by distillation.
On another note: if you had everything, wouldn't you have to keep it everywhere? (Forgive me; it's been a long day  ) ) |
Good point. (about everything)
About the column I'm guessing it's 15, since it has 15 of those little trays in it. But what I don't know about Oldershaw columns you could almost
cram into the Astrodome.
I figure I'll try it out with a variable reflux head and some insulation. But to be frank I have no Idea if there's any peculiarity to how these are
used as opposed to like a Hempel column.
It should arrive next week. It's 29/42, and it's about 600mm long.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Sweet piece of glass there SWIM. It is a design I have not seen before. How is the Oldershaw functionally different from a Snyder column? It does
look a lot more compact and lacks the fun moving parts.
I too would guess 15 theoretical plates for your purchase. And I woud surmise that it would start doing strange things if it ever began to get
flooded.
It seems you do have the Midas touch.
(The misers' touch is if you don't share it. The minus touch is when you drop it. And the miners' touch is if you are doing your chemistry
underground.)
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Here are a couple of papers I have, but honestly I haven't read. Maybe they will be some help concerning HETP. I thought I had a clearer explanation
somewhere, but can't find it now. It may have been in Fieser's Org. chem lab manual, or maybe in Vogel. Both of those books are in the forum library,
I think.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Nice. Those columns are super pricey!
I got a second 30 cm Vigreux from Deschem today (Deschem sells nice Vigreux columns). But unpacked, I doubt that the combined separating power of all
of the columns of my humble lab have the separating power of that Oldershaw column.
I also got a couple of jointed quartz tubes. I'm going to wrap one with copper tubing and use it as a condenser and use the other one as a combustion
tube. Boron tribromide is theoretically within my grasp.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I have a few various jacketed beakers and fritted funnels/reactors if people want any. I think the beakers are 100 to 400 ml, and the fritted
reactors span tiny (1-2ml?) to medium ones, not really sure, most look custom or are unlabelled. But I am happy to sell them inexpensively, since
they would take a while to list on Ebay. I'll try to get some photos one of these days, but they are mostly scattered in a few boxes of random
things.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ok, not glassware but an interesting equipment purchase nonetheless.
At a garage sale today I scored about 15kg of lead for five bucks. There were two buckets of lead head nails, some lead bars and wires, some lead
roofing flashing and this gem -- a home-made lead beaker. I don't think it will inspire me to do the lead chamber process but I can see it being
useful for some electrolysis where I want a lead anode.

I also scored six glasses and a jug in what I believe is depresion-era uranium glass. Also five bucks. I have yet to test it though.
(Not sure whether this belongs here or in the repurposed/homemade lab gear or chemical purchases thread. Guess it is here now.)
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I snagged three nice reagent bottles today from my university's dumpster. They were throwing out several empty Sigma Aldrich and Fisher bottles,
mostly for alcohols and ammonia. I will go back for more but I could only fit so many in my backpack.
|
|
|
WangleSpong5000
Hazard to Others
  
Posts: 129
Registered: 3-11-2017
Location: Oz
Member Is Offline
Mood: Curious
|
|
Just received a lovely 1 litre 24/29 Erlenmeyer flask. Also finally recieved my glass spirit thermometer... which I sat on within a day. Ffs...
Hyperbole be thy name
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Got this little spinning band still today.
Just couldn't pass the damn thing up.
It's supposed to have 5 theoretical plates to the inch, so 12 or 15 total.
It's also supposed to have a 90 minute boil-up time, and that air condenser around the thermometer probably gives the whole thing quite a reflux
ratio.
So distilling 4ml of something with this may take quite some time.
Anybody familiar with these?


|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Wow, that is super cool. I wouldn't have passed it up either.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Some little swizzle sticks that seemed like something nice to have for art or science, the total price a little under 8.6 cents per swizzle. What
might be a typical use for these I wonder?
[Edited on 6-4-2018 by Morgan]
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by SWIM  | Got this little spinning band still today.
Just couldn't pass the damn thing up.
It's supposed to have 5 theoretical plates to the inch, so 12 or 15 total.
It's also supposed to have a 90 minute boil-up time, and that air condenser around the thermometer probably gives the whole thing quite a reflux
ratio.
So distilling 4ml of something with this may take quite some time.
Anybody familiar with these?
|
Could you maybe replace the pics with some in focus? There are some small details that are of interest. Nice snag, did you find it locally or online?
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
Quote: Originally posted by RogueRose  |
Could you maybe replace the pics with some in focus? There are some small details that are of interest. Nice snag, did you find it locally or online?
|
Look up a Hickman-Hinkle still.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Morgan  | Some little swizzle sticks that seemed like something nice to have for art or science, the total price a little under 8.6 cents per swizzle. What
might be a typical use for these I wonder?
[Edited on 6-4-2018 by Morgan] |
I don't know what the typica use is. They will make good sleeves for thermocuples and heating element forms.
Are you in the UK or Europe, if so do you want to sell say ten of them and for how much?
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48  | Quote: Originally posted by Morgan  | Some little swizzle sticks that seemed like something nice to have for art or science, the total price a little under 8.6 cents per swizzle. What
might be a typical use for these I wonder?
[Edited on 6-4-2018 by Morgan] |
I don't know what the typica use is. They will make good sleeves for thermocuples and heating element forms.
Are you in the UK or Europe, if so do you want to sell say ten of them and for how much?
|
I'm in the U.S.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Having that much quartz seems like it should be useful for something.
Have you tested them for thermal shock?
How difficult is it to actually melt the end to make a thermocouple sleeve?
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
The thought crossed my mind, but in the process of getting settled in a new house my torches aren't at hand. I wrote a company in the Netherlands late
Friday to find out what the glass tubes are and what they might commonly be used for. It would be sad if they are just ordinary glass.
So just now it dawned on me a small handheld butane torch was in a box in the other room. What unfolding drama the little experiment was. Using a
small wide glass of water, the mystery tube was heated above the water at the point in the 5 cm long flame. My heart sank when the blue flame started
showing the typical signs of a sodium flare. But upon further heating the yellow faded and disappeared. Apparently wiping any oil or salt from
handling on your shirttail is hardly the way to go of course, but the main test was going to be the thermal shock. And recalling how quartz lightbulbs
have the warning not to handle them with your fingers, that oil or salts could damage them, that came to mind too.
The grand moment came when the hot end of the tube was submerged in water. Now I've dunked some 25 mm diameter Heraeus fused quartz in water and the
sound it makes can be misleading, as if it might have cracked but this little 4 mm tubing not only sizzled and popped, it made a distinctive ping and
it was heartbreakingly assumed to have surely fractured, but happily the factory flame polished end was still intack when removed from the water, yet
disbelievingly so after first looking for fragments in the bottom of the glass.
Seeing some segments of water trapped or taken up in the tube from the test, it occurred to me one use, albeit meager, would be to craft a little
putt-putt boat, the
tubing as good as metal in that respect.
https://sciencetoymaker.org/putt-putt-boat/patents-for-putt-...
On burping ...
"That was just the start of it. He set out to find out why some engines--even those without leaks--go dry and stop working. This is known as "burn
out." It is very frustrating and mysterious."
https://sciencetoymaker.org/putt-putt-boat/putt-putt-boat-in...
[Edited on 8-4-2018 by Morgan]
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | Having that much quartz seems like it should be useful for something.
Have you tested them for thermal shock?
How difficult is it to actually melt the end to make a thermocouple sleeve? |
I haven't melted any quartz but was able to only slightly deform a piece of 10 mm tubing one time with just a Mapp gas torch that mixes in air. Maybe
a 4 mm tube would have a chance being smaller and more likely if you could surround the flame in fire brick or something perhaps. Quartz emits UV when
working and I don't have any eyewear for that.
The coe of the thermocouple wire I guess would match that of quartz - if made for mating the two.
[Edited on 8-4-2018 by Morgan]
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Well despite the posts best effort my new parts arrived. thanks to deschems excellent packers!
   
[Edited on 11-4-2018 by XeonTheMGPony]

|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Yowzer!
It's a while since I have seen a postal parallelogram as good as that one.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Morgan  | Some little swizzle sticks that seemed like something nice to have for art or science, the total price a little under 8.6 cents per swizzle. What
might be a typical use for these I wonder?
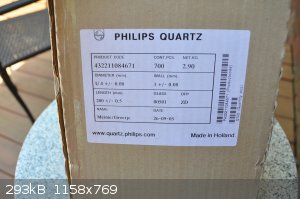
More information ...
I contacted the eBay seller today and he wrote back this.
"I believe that the quartz tubes were meant to be used to make custom HID lamp bulbs.""I'm curious what you might be using them for though..."
I wrote him back and asked him the same and how he came about them. He said he had more.
I contacted Philips quartz and the representative said they were possibly the internal tube of a quartz bulb, the woman sounded like a new hire and
couldn't find out anything more from the product code. I spent about ten minutes talking to her just to gather that scant information.
[Edited on 12-4-2018 by Morgan] |
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
The quartz tubes that were sold to me were going to be used for a flashlight. The seller replied "The custom bulbs were going to be for a special
flashlight built for the military. They had to be custom made for the level of brightness and IR output required. "
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
eBay just gave me a $20 off coupon... I'm giving some serious thought to these:
 
|
|
|
greenlight
National Hazard
   
Posts: 753
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Oooh thats a nice pressure equalizing funnel, haven't seen one with two stopcocks before.
Be good, otherwise be good at it 
|
|
|
| Pages:
1
..
8
9
10
11
12
..
20 |