| Pages:
1
..
4
5
6 |
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Quote: Originally posted by sargent1015  |
I should have clarified, while it is cool and dandy to make copper benzoate, I do not need a huge surplus of it. So I made the benzoic acid, isolated
it, and used only the amount I wanted for the copper benzoate synthesis.
|
I see, very nice. Did you use hardware store toluene? I want to do this but I don't really want thiophene containing wastes associated with the
purification, as i don't know how to destroy it/ dispose of it. Any ideas?
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Actually, yes! It was hardware store toluene, I even took a 400 MHz NMR after the synthesis and it was remarkably pure.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Also, toluene can be cleaned up with a couple sulfuric acid washes (Enter Vogel reference here). Did you try the synthesis yet?
Well, I also have some pictures since everyone loves that.
First, this is the aspirinate/imidazole complex (Maybe?). It is very waxy and a deep, dark blue.

Second, a little copper benzoate!

|
|
|
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
In this thread: http://www.sciencemadness.org/talk/viewthread.php?tid=26316 a paper is given on the synthesis of aspirinates of copper, nickel, cobalt, and zinc
(Journal of Enzyme Inhibition and Medicinal Chemistry, 2002, Vol 17 (2), p 87-91). It's another lousy paper, riddled with errors.
First of all, it gives exactly one synthesis, that of a cobalt aspirinate, and declares that the other compounds were made by the same procedure, only
with different molar ratios of the reactants. Insufficient experimental data!
Second, the formulation for the compounds are given as ML2, MCl2L2, and M2L4 (where L =
aspirinate or acetylsalicylate). Since these are divalent metals, the formulation with two chlorides and two aspirinate anions is clearly wrong (that
would require a tetravalent metal).
Thirdly, how on earth is one supposed to get one of these three complexes through "different molar ratios of the reactants", when there are two
aspirinate anions for each metal in every single proposed formulation?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Well hi there guys! I have not been working on anything for this forum in quite some time since I moved across the country and started grad school in
organic chemistry. Not only did I not have the space to haul all of my equipment, but my time outside of lab anyways is quite limited, so I will live
vicariously through you guys!
Has anyone done asprinate work in awhile? Maybe I can revitalize this thread and get someone to pick up where I left off 
Anyways, hope everyone is doing well 
|
|
|
Kitsune1
Harmless

Posts: 19
Registered: 10-3-2015
Member Is Offline
Mood: No Mood
|
|
I have around 10g of ASA in my lab ready to extract when I find time so I would be interested in trying acetylsalicylate metal complexes; after I
finish my current project, Nd extraction with organic ligands.
I do have to say my preferred method of ASA separation is a bit different; after dissolving the tablets in an appropriate volume of warm ethanol,
filter and add the same quantity of distilled water (the ASA crystals precipitate which is really pretty), let the crystals settle. Decant into a
filter, wash with cool water and then leave to dry (don't evaporate with heat, this ends with thermal decomposition).
A benefit to this is that the ethanol can then be distilled and used again and all named binders (in my source at least) are insoluble in ethanol, the
efficiency is also very high due to the insolubility in low concentration ethanol.
|
|
|
aaronperezb
Harmless

Posts: 2
Registered: 22-4-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bfesser  | | I replicated the experiment shortly after that post, substituting 0.9 mm pencil lead, and got very poor results. I did get some product, but the
crystals are much smaller than claimed by the original authors—I didn't bother to recover them. |
|
|
|
aaronperezb
Harmless

Posts: 2
Registered: 22-4-2015
Member Is Offline
Mood: No Mood
|
|
Please use drinking water to improve your results and you can optionally replace the pencil lead by a copper wire as cathode. Interrupt the power
supply when a black powder (CuO) begins to appear but let the chemical reaction finish, this means the blue powder (Cu(OH)2) disappears.
|
|
|
Muhammad
Harmless

Posts: 6
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
So I followed the steps for the synth of copper acetylsalicylate. I didn't get an immediate formation of precipitate, just a formation of a greenish
blue solution. I checked an hour later and crystals were forming on the sides and the base. The crystals are insoluble in water, have and greenish
bluish hue and And slightly remind me of tiny acetate crystals. Have I done it right?
|
|
|
Muhammad
Harmless

Posts: 6
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
Copper (II) acetylsalicylate precipitation
I did a precipitation with copper II sulfate and sodium acetylsalicylate and the product did not precipitate immediately. instead it crystallized on
the walls of the container over some time. Was this precipitation? I do not understand what happened.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Try posting your entire procedure, sources, pictures, and how your prepared the reagents in your reactions. Your post is not detailed enough. IF you
can read it in 30 seconds its most likely not worth posting.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
Texium
|
Threads Merged
10-9-2017 at 14:33 |
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
So did anyone manage to get an aspirinate other than that of copper? We've got cobalt(III) and iron(III) mentioned in the thread, but were these
followed up to be sure that they were actually the desired compounds?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I just tested a solution of aspirin, dissolved in acetic acid and adjusted to a pH of 7, with cobalt chloride and manganese chloride- no precipitate
was observed.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Hector
Harmless

Posts: 8
Registered: 25-10-2017
Member Is Offline
Mood: No Mood
|
|
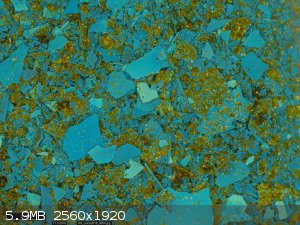
after crystalising, the copper aspirinate deposited on the walls of the flask, as it can be seen from nice smooth shards. however, we got these
goldish needle-like crystals that don't really fit any description.
the synthesis was done with copper sulfate and pure ASA
an EDS analysis was done and it shows the blue aspirinate and the needle-like golden crystals to have a somewhat similar composition (the gold needles
contain less copper and more organic)
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
I also had a go at copper aspirinate, and then did some test tube checks with the metal salts I had available to see if anything else obviously reacts
with sodium aspirinate. The copper first:
- First step to make the sodium aspirinate in solution:
Na2CO3 + 2C9H8O4 = 2C9H7O4Na + CO2 + H2O
- 4.7g NaHCO3 (baking soda from the local department store) was dissolved in 50ml warm water, obtaining a clear solution.
- Add 10g aspirin powder (lab reagent quality, bottles says "Aspirin") in stages to the stirred solution. Bubbles followed each addition, wait for
bubbling to subside before adding more. All 10g dissolved, plus an 11th gram, but the 12th gram did not all dissolved. Filter the solution to remove
the unreacted aspirin.
- 6g CuSO4.5H2O in 20ml water, heat a bit to fully dissolve. Slowly pour the CuSO4 solution into the sodium aspirinate solution to get the double
displacement:
4C9H7O4Na + 2CuSO4*5H2O = C36H28O16Cu2 + 2Na2SO4 + 10H2O
- Stir for some 20 minutes, dark blue solution. Photo below.

- Vacuum filter and wash once with excess of cool water in the funnel. Photo below of the wet remainder. The filtrate had a very pale magenta color.

- Dry remainder on steam bath till weight reduction slowed to near nothing. Dry dark blue powder, 10g recovered. Photo below.

I then made another solution of sodium aspirinate by using the same procedure as above. I dissolved various metal salts that I have in the shed one by
one in a bit of water in a test tube and added a bit of the sodium aspirinate to see if there was an obvious ppt. Observations:
- MnSO4 - no obvious reaction.
- (CH3COO)2Co - no obvious reaction.
- Cr(NO3)3 - no obvious reaction.
- Ce(NH4)4(SO4)4 - dark olive color ppt.
- (CH3COO)3Er - nothing.
- FeSO4 - solution turns dark brown color.
- Fe2(SO4)3 - solution turn very dark purple color with brown ppt.
- Ni(NO3)2 - no obvious reaction.
- TiCl3 - brown ppt, difficult to remove from test tube.
- SnCl2 - light yellow ppt, but when I retried on a larger scale I got a white very sticky mess.
|
|
|
| Pages:
1
..
4
5
6 |