| Pages:
1
..
57
58
59
60
61
..
77 |
plastics
Hazard to Others
  
Posts: 141
Registered: 6-11-2009
Member Is Offline
Mood: No Mood
|
|
https://www.sciencemadness.org/whisper/viewthread.php?tid=89...
Tried it myself ages ago

|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Very nice guys, wish I had more time for organic synthesis...
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Aluminium Copper Alloy

|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Nicely crystallized, could you please provide more info?
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Im trying to make raney copper and this was seen on a blob of 1:1 Al:Cu metal that was cooled by pouring into water. The picture was taken through a
loupe but im still impressed by the crystal size considering the cooling rate. It is probably Al2Cu .
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
It is beginning to get very cold where I live. I was doing an inspection of my chemicals (to make sure no glass bottles broke due to freezing) and was
pleasantly surprised with this. The water crystals in this have formed nice, needle like structures in the bottle.

|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Mandelbrot set
View my zoom movie into the Mandelbrot set. I trimmed down from 150MB
Attachment: trip5.avi (6.9MB)
This file has been downloaded 911 times
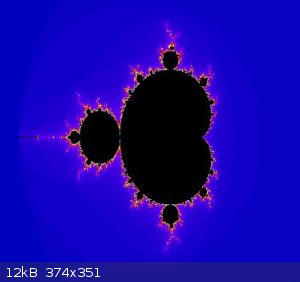 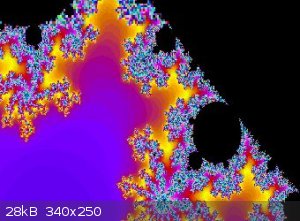

[Edited on 19-1-2018 by wg48]
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Got a small vial of trimethoxy benzaldehyde some time ago, extra toss in from getting indium sample. Well, I found it yesterday when cleaning.
KA-THUMP! What was that... Ohh just a pile of stuff put in an odd location months ago.
Any how it was brown and odd looking. Got to check the melting point sans thermometer, = melts under boiling water temp. But only gave back brown
looking crud. Did again and left wrapped in a towel to cool, big plates but not exactly better, same appearance. But it was soluble in water to a
minor extent and I just used that capacity. Made a nice sat solution while learning the fun of makeshift filter and clogging said filter.
But the results were at least aesthetically pleasing, even if grueling to do. The day job suffered a touch from low sleep  science when you can, consequences be damned. science when you can, consequences be damned.
 
Crystals <------> crude mid solidification
 
Suggestions for a legit use?
[Edited on 20-1-2018 by violet sin]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Oh, hey, I know something about that.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
You sure do man, it was almost lost to a crash of phosphoric acid, oil of wintergreen, Cu/NH3 cellulose solution, and some other random crap(not
headed to garbage) left in the gutted out microwave that is FINALLY heading to the dump. Everything lived some how even after getting briskly lifted
and tossed in a truck, lol.
Here are a couple pics from actual time in workspace. I was trying to study up and identify positively rhenium powder from an eBay purchase. It did
not like most of the chem I threw at it. The borax bead test worked though. Sulfuric acid, not so much. Hydrogen peroxide 30% dented it though, add
in some ammonia and it was dissolving the dust. And a last test, a tiny bit heated in a test tube with air in there made a subliming straw yellow
oxide. Missing some pics though...
Ammonium perrhenate (1-4)
   
Ammonium tungstate and a super tiny mag stir w/ Ru powder
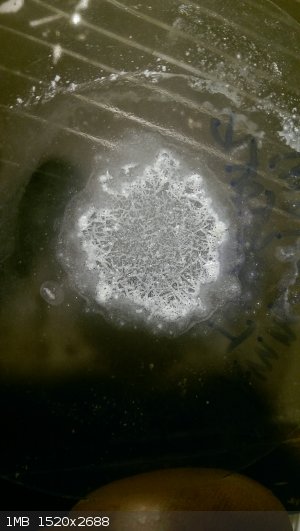 
We should come up with a "testing eBay buys" thread for frustrated purchasers. Man that would be useful, lost ~230$ a couple years back on fake Or
powder... But if you figure it out soon enough, eBay covers it.
[Edited on 20-1-2018 by violet sin]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Drinking too much is bad for the health, however Nature makes use of Everything.

|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
That's an example of green chemistry, isn't it?
|
|
|
WouldSynthesizeForFood
Harmless

Posts: 8
Registered: 19-2-2018
Location: Ukraine
Member Is Offline
Mood: No Mood
|
|
Some photos of a mixture of p-xylene and water from who knows how long ago:
 
[Edited on 19-2-2018 by WouldSynthesizeForFood]
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
1)Thrift store find, another good book.
2) EBay purchase 5$ = 50 switches.
 
 
3) Can't really tell, but that's bees wax bubbling up outta yuck soup... Rotting pollen etc. from a failed colony.
4) Built another ATX power supply for working around with small projects. The two power resistors are fixed on a heat sink over the exhaust fan,
conveniently blowing outward.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Whoops, might’ve left some sodium sulphate solution in a separatory funnel. Also, a sodium sulphate forest in a 250mL RBF.
  
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
RogueRose
International Hazard
    
Posts: 1596
Registered: 16-6-2014
Member Is Offline
|
|
I think this is a CaSO4 crystal in a solution of HNO3 and H2SO4. I took a concentrated solution of Ca(NO3)2 and added it to a 30% solution of H2SO4,
with excess H2SO4, filtered and then tried to distill off some of the HNO3 (didn't work to well) but about 150ml of liquid came over, so there was
more CaSO4 dissolved in the hot solution after about 2 weeks I checked the bottle and found these little beauties growing.
  
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Wow those are beautiful.
Is it possible to take them out of the bottle or are they too britle?
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by violet sin  | We should come up with a "testing eBay buys" thread for frustrated purchasers. Man that would be useful, lost ~230$ a couple years back on fake Or
powder... But if you figure it out soon enough, eBay covers it.
|
I too have had difficulty fiding pure Or powder 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
On the last page, I had a similar thing going on with the fluffy crystal balls. They’re far too delicate to do anything with and just disintegrate
when touched, which is disappointing to say the least. Best thing to do would be to transfer the solution into a fresh ‘display’ container to let
it evaporate off, where it can then be capped and stored (not sure how they behave with long exposure to air, but I’ve grown some stunning crystals
in the past just for them to fall apart into powder!)
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
sulaiman: ya got me there... Ir as in Iridium... 99% of the time I'm typing on a phone that was popular in, wow I'm not sure. wiki- "The smartphone
was unveiled on 19 February 2013". I've been a victim of the dreaded Auto-Correct, no matter how it occurred  i blame the operator i blame the operator
|
|
|
Vicarious3rdEye2
Harmless

Posts: 20
Registered: 3-2-2018
Member Is Offline
Mood: No Mood
|
|
Not quite sure what this is but it is beautiful
I ran a cell using two graphite electrodes in my first attempt to make NaBrO3 from NaBr and after putting the solution after electrolysis into a
beaker and added some KOH in the hopes that the less soluble KBrO3 would crystallize out I ended up with this.

|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That looks sweet. It's always fun to go down to the lab and find something like that, even when you're not 100% certain what it is.
|
|
|
Vicarious3rdEye2
Harmless

Posts: 20
Registered: 3-2-2018
Member Is Offline
Mood: No Mood
|
|
Yeah I know right?
I left the solution sitting in a beaker for about three days until I figured out a way to get the graphite powder out of solution without using a
glass fritt and then saw this sea urchin looking crystal and decided it was just too pretty to dissolve it again.
I actually had another strange formation, I did the procedure for p-DDNP from acetaminophen nitration written by I believe the member nitro-genes
which was an excellent and fun project. I was recrystallizing recovered Isopicramic acid before diazotization and I had it sitting in a weighing boat
dissolved in MeOH and it seems that as the solvent evaporated it grew these strange looking crystals

|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Yellow Cardinal
https://www.youtube.com/watch?v=0TrcQMz2lnk#t=1m9s
[Like its red counterpart, this rare cardinal relies on the carotenoids (organic pigments) in its diet to turn its feathers a bright yellow. But diet
isn't everything: Research has shown that certain genes determine which of several carotenoids the bird deposits into its feathers and bare skin.]
[For instance, red cardinals synthesize their red hues from four yellow or orange pigments they consume, according to research published in the
journal The Condor in 2003.]
[In that study, researchers found that the plumage of a yellow Northern cardinal collected in 1989 in Baton Rouge, Louisiana, didn't show any of the
red carotenoids found in common Northern cardinals. Assuming the yellow bird had access to similar foods as the red-hued ones, the researchers
concluded that this bird couldn't manufacture any of the four carotenoids typically found in a cardinal's red feathers. A genetic mutation, they said,
knocked out the bird's ability to carry out the chemical reactions that would have led to red feathers.]
https://www.livescience.com/61897-rare-yellow-cardinal-alaba...
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
A not very natural scenery
Im making a lot of Copper Acetylide for an upcoming project, this is a mixture of Cupric Chloride and Nickel Chloride that i have lazily let dry out
over a couple of weeks before i separate it. It grew some nice structures and with the blue wall of my lab it looks like some kind of scenery.

|
|
|
| Pages:
1
..
57
58
59
60
61
..
77 |