| Pages:
1
2 |
5Qr25Pla
Harmless

Posts: 11
Registered: 18-2-2018
Member Is Offline
Mood: No Mood
|
|
LiH Production Apparatus Design and Operation
this crudely drawn apparatus is meant to produce hydrogen (from the left) and then cool the hydrogen and dry it then heat the hydrogen again and pass
it over scored/cleaned lithium metal in a reaction vessel.
before the reaction takes place the vessel is purged with hydrogen.
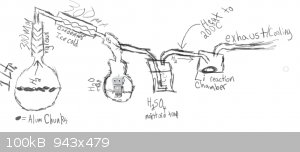
part - 1 - Hydrogen
The hydrogen is produce from the reaction between water and aluminium and is catalyzed by NaOh. In trials, i was able to successfully produce hydrogen
for up to 3 hours with no sign of slowing in a 1 litre reactor after 3 hours the hydrogen production began to slow significantly but i believe this is
because of over-saturation of aluminium hydroxide in the water and not so much because of a decrease in catalytic activity from the NaOH.
part - 2 - Drying the Hydrogen
The reaction between the aluminium and the water is quite exothermic with higher quantities of NaOH especially (otherwise though, not enough h2 is
produced) and because of this there is an exess of moisture in the vapors coming from the reaction vessel (not to mention very fine particles that are
carried by this moisture/vapor). in order to dry the hydrogen and prepare it for reaction with the lithium (as moisture will react with the lithium)
it is first directed through a vigreaux/air cooled collumn (to keep as much water in reactor as possible) and then through a distillation adapter and
into a coil-type condenser that has been cooled by ice cold water. more moisture is condensed here and is collected in a water trap. after the water
trap the vapors travel onwards out through a vaccuum adapter and into stainless steel tubing.
part - 3 - Further Drying the Hydrogen
the stainless steel tubing directs the hydrogen into a trap filled with (currently h2so4. but this is probably not a good idea as if it successfully
traps h2o it should release SO2 ??) this is only a precautionary step though as the hydrogen seems to be dry enough already. This step needs to be
improved. i need something to dry the h2 and preferably also remove any residual O2 if possible.
part - 4 - Heating the Hydrogen
in this step the tube that the hydrogen is in is heated just before a thermostat. the hydrogen is heated to 200C
part - 5 - Reaction
the hydrogen is reacted with lithium to produce lithium hydride (in a stainless steel reactor that has a top that has an inlet and an outlet and a
bottom that comes off)
note: the lithium is scraped on the surface to expose fresh lithium just before reaction.
note 2: in the first experiment the hydrogen was not heated as i am worried about the moisture level of my hydrogen. The reaction was mostly a failure
as the lithium was only barely scratched before the reaction but where it WAS scratched was covered with a powder that was easily distinguished from
the oxide/nitride/etc layer that normally forms.
note 3: the lithium might need to be cleaned while covered by diethyl ether and then dried by the heated hydrogen for best reactivity. but i am hoping
that this is not the case. any info on the reactivity of hydrogen and lithium would be appreciated. im sure its out there. but i havnt looked hard
enough i suppose  ill keep looking though. ill keep looking though.
Question: how can i dry my hydrogen.
Question 2: moisture seems to be the biggest danger in this experiment and i am confident that the moisture level is really low after the coil
condenser already. this being said, would a more experienced person be brave enough to raise the temperature of the hydrogen to say 180C - 200C and
attempt to react the hydrogen more quickly/efficiently?
if success happens: i plan to keep it in the reaction chamber (for only a few minutes) under hydrogen and then either attempt to dissolve it slowly in
anhydrous ether mixed with aluminium chloride (for storage in the form of LAH) or dispose of it as i am truly afraid of chemicals that can ignite so
easily. even if it's not likely.
in the future: if i am successful i hope to share my findings and experiment to find reliable methods for hydride production so that others may
benefit.
coil condenser shown below

[Edited on 19-2-2018 by 5Qr25Pla]
[Edited on 19-2-2018 by 5Qr25Pla]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I suggest you put a condenser (a different one than shown in the pic) on top of the flask and add a addition funnel to allow control the gas
generation. Then you will not need the column or the first water trap.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I highly recommend that you read the sections on making LiH in "Small-Scale Synthesis of Laboratory Reagents," a book by Leonid Lerner (known as Len1
to veteran forum members).
This is a valuable synthesis which could lead to LiAlH4, a very useful reductant. I wish you the best of success.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
An excellent pre-write up, good luck when it comes to carrying out the procedure, certainly not something I’m willing to try at this stage. For
drying the hydrogen gas, pass it through a drying tube packed with desiccant: gas generator -> rubber tubing -> bung with glass tube ->
drying tube -> reaction vessel (see image for example). From what I understand, hydrogen gas and concentrated sulphuric acid shouldn’t react so
bubbling it through is a good way to remove moisture.
Just to clear up a misconception, NaOH doesn’t act as a catalyst as it is consumed by the reaction, producing sodium aluminate and hydrogen. 2 NaOH
+ 2 Al + 2 H2O -> 2 NaAlO2 + 3 H2.

In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
5Qr25Pla
Harmless

Posts: 11
Registered: 18-2-2018
Member Is Offline
Mood: No Mood
|
|
@LearnedAmateur
thank you, that image was very helpful. and knowing that the sodium is used up in the reaction makes me wonder if it would be better to put more
sodium.
@wg48 already mentioned that an addition funnel for adding more liquid/naoh mixture would let me add more liquid over time without letting other gases
in. so i added an addition funnel to the apparatus. this should clear up the problem of gas production slowing down.
@Magpie
i couldnt get my hands on a copy.  is it in the library this site has by chance? or
someone else's maybe? is it in the library this site has by chance? or
someone else's maybe?
My main concern here is how reactive/volatile/powerful the reaction between the lithium and hydrogen when i increase the heat. knowing what the worst
case scenario is would be very helpful. the amount to be processed is 1 gram or less.
the apparatus will be purged by produced hydrogen before any lithium is introduced to the reaction vessel. this should eliminate all possibility of
flashback if something goes bad. potential pockets of oxygen/nitrogen/etc... will be eliminated in final build of apparatus.
other info: the heat is supplied by NiCr coil + blower + potentiometer + thermostat at end of blower to ensure heat doesnt exceed 392F/200C (supposed
autoignition temperature of Lithium Hydride according to https://cameochemicals.noaa.gov/chemical/996.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Leonid Lerner's book is available through the CRC publishing company. It has many valuable syntheses. I used one for making CCl4 from chloroform
with nearly 100% yield.
If you read the experimental section on making LiH you will see that it is not trivial.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Quote: Originally posted by 5Qr25Pla  | | ... knowing that the sodium is used up in the reaction makes me wonder if it would be better to put more sodium. |
Your best bet would be to calculate how many moles of aluminium and sodium hydroxide you’ll need from the equation I provided, and then add another
5-10% to account for losses and the initial purge, as well as the aluminium oxide layer which won’t produce hydrogen. You shouldn’t need to figure
it out for the water though, that should be in excess anyway as the solvent for the NaOH.
Just out of curiosity, what will be your source of Al? Powder? Turnings? Foil? That will have to go into your considerations for H2 yield, as the
larger the surface area, the higher the proportion of Al2O3.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
moisture seems to be the only danger in this experiment and i am confident that the moisture level is really low after the coil condenser already.
this being said, would a more experienced person be brave enough to raise the temperature of the hydrogen to say 180C - 200C and attempt to react the
hydrogen more quickly/efficiently?
|
You have a bit more than moisture to worry about here. Hydrogen is an escape artist, with a wide range of explosive mixture in air.
Handling the ammount of Hydrogen required to synthesize a moderate quantity will quickly show just how good your planning and technique (particularly
safety) are-
The strong base, strong acid and flammable reactive alkali metal/whatever product has formed may well come out and join the party too, after a
Hydrogen explosion.
|
|
|
5Qr25Pla
Harmless

Posts: 11
Registered: 18-2-2018
Member Is Offline
Mood: No Mood
|
|
my aluminum is in the form of whole melted but coarse on the ouside chunks. these were produced by melting aluminum from other sources and then heated
extremely hot till brittle then poured into crude "ingots". these ingots float in my liquid well and produce a lot of hydrogen for quite a while (one
ingot produces a flow of hydrogen for about an hour) the length of time of reactivity is adjusted by the surface condition of the aluminum and the
density. smoother/denser aluminum "ingots" react slower at first due to the smooth edge and then react longer due to density. the much less dense
aluminum chunks are very rough and react very quickly and vigorously. these react for the first 10-15-30 minutes while also making the water very
warm. this purges the system and gradually as the water warms it activates the activity of the other, more dense chunks of aluminum which then carry
the reaction to completion. (about 2-3.5 hours as prep-chem states that 2 hour residence time is more than is needed at 125C)
In the post titled: "lithium hydride synthesis" the user @JJay says that "Small Scale Synthesis of Laboratory Chemicals suggests heating.... ...to
700C". Is heating lithium to 700C really necessary to ANY degree?. www.PrepChem.com suggests that this exact reaction will proceed "at temperatures as low as 29C" with a 60% yield at 99C and an 85% yield at 125C.
to me. it seems like no more than 150-250C is necessary.
the main factor contributing to difficulty of producing the hydride is the surface condition of the lithium. if this is the biggest problem to
overcome couldn't one potientially just scratch the surface of the lithium while in a hydrogen/argon atmosphere and then start the reaction without
ever letting oxygen/nitrogen into the picture?
Worst case scenario, the melting point of lithium is ~180C. The melting point of LiOH is 462C. This "could" be bad in certain circumstances where the
lithium is protected by the N/O/x layer, keeping it from reacting. But wouldnt the lithium just melt away from the O/N/x layer and then the lithium
would make LiH and the LiOH would make MINUTE amounts of H2O which would then partially react with the lithium again and partially would exit via the
exhaust. Eventually the OH would be almost entirely removed, giving way to the purer/smoother production of LiH.
If there IS an explosion it should start at the lithium reaction chamber and vent through the exhaust possibly leaving a burning flame of hydrogen
(which could be avoided by simply burning the exhaust anyway)
The explosion produced by 90% Hydrogen 10% Oxygen (worst case scenario. Less than 1% oxygen is expected) doesnt seem to produce much force. This
sounds crazy. But in a glass bottle it did not burst the bottle upon ignition. I am wondering if ignition happened would maybe good glassware handle
the ignition? Im thinking some probably could handle it easily. Once ignition happens the spare o2 would be used up immediately and the rest of the
reaction could be considered "safe" (unless there are ungodly amounts of LiOH in the reaction chamber. But again, im only using 1 gram for analytical
(and practical) purposes.
The exhaust port is larger than the intake port of the reaction chamber encouraging expansion in the direction of the exhaust.
The entire apparatus is completely sealed before start-up using vacuum grease for ground glass adapters and is placed outside of the lab to avoid
hydrogen buildup. The reactor is also outside. all tubing/adapters are also sealed. the heat of the lithium reaction vessel is kept AWAY from the
hydrogen production vessel and there is a spiral tube between the two vessels to create further friction and reduce momentum in the direction away
from the lithium and towards the Al/Naoh/H2O mixture (so as to hopefully avoid bursting if explosions of any size happen)
the hydrogen production vessel is controlled and tended to by another "hobby technician" so that complete focus can be had on the subject of heating
the hydrogen "safely" below its autoignition temperature of 500C and ensuring that Hydrogen leaks ARE NOT HAPPENING.
I am wondering what the likelyhood of autoignition of hydrogen is in a "pure" and sealed environment. i am thinking that if the apparatus is sealed
properly the chance for autoignition is essentially 0% (but probably more realistically like 0-5% considering the possible ignition of pockets of
produced H2O [from LiOH + LiH ---> Li + H2O?])
I am also wondering about how exothermic this reaction might be. is there a risk of it heating to above 250C on its own somehow?
[Edited on 19-2-2018 by 5Qr25Pla]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
The explosion produced by 90% Hydrogen 10% Oxygen (worst case scenario. Less than 1% oxygen is expected) doesnt seem to produce much force. This
sounds crazy. But in a glass bottle it did not burst the bottle upon ignition.
|
How did you come up with this as worst case? You are not using O2, but air for an environment.
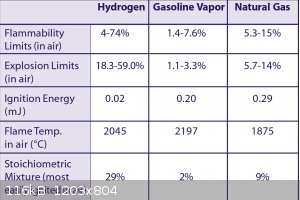
|
|
|
5Qr25Pla
Harmless

Posts: 11
Registered: 18-2-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  |
| Quote: |
The explosion produced by 90% Hydrogen 10% Oxygen (worst case scenario. Less than 1% oxygen is expected) doesnt seem to produce much force. This
sounds crazy. But in a glass bottle it did not burst the bottle upon ignition.
|
How did you come up with this as worst case? You are not using O2, but air for an environment.
|
i dont think i have been clear enough  i am sorry. let me clarify. i am sorry. let me clarify.
the ENTIRE apparatus (including lithium reaction chamber, exhuast and heating tube) is PURGED with hydrogen or argon (hydrogen in my current case)
prior to initiation. when the apparatus has been purged with (roughly 3 litres @ 1atmosphere) enough hydrogen/argon then the lithium is placed in the
reaction vessel with the hydrogen still running. when the lithium is in the reaction vessel another 0.5 - 1.0 litres of hydrogen are used to purge the
(TINY) reaction vessel. once the lithium has been introduced and the hydrogen has purged the system, then and ONLY then is heating to begin. the
heating is done by the hydrogen itself so as to avoid hotspots on the lithium and to increase the reactivity of the hydrogen.
the picture that you @Bert provided was useful to me though. if the vessels have been purged properly (and there are no air pockets available for
other gases to dwell in) then there should be relatively no risk of fire/explosion during NORMAL operation (ASSUMING NO LiOH/Lixx is present. but. as
there will almost for sure be LiOH/Lixx present there is still the very small chance that the reaction produced between the Li, Lixxx, LiH, H2, and
any produced gases could create small, localized, explosions.
these "explosions" should be relatively harmless actually as long as the Lixx % is kept very low (preferably at < 0.05 grams total impurity or
less) and the reaction is kept slow/low temp.
the reaction doesnt need to yield %100. and even at room temperature the yield should be "enough"
the added heating is PRIMARILY used to greatly reduce the reaction time (allowing the reaction to complete before the hydrogen stops being produced)
but making the yield a higher percentage is also a very nice benefit. this will also help to keep the LiH from getting trapped in unreacted lithium.
i have ran the apparatus with very slight success. but with no heating. i am afraid that when the reaction really gets going it will be unstoppable
due to the heat that it generates. a good example of a reaction that gets going and then heats itself and continues to heat itself is the reaction of
methanol over a red-hot catalyst. the methanol goes over the catalyst and then reacts with minute levels of oxygen and produces formaldehyde. the
reaction is so hot that it will sustain itself as long as the gases are pushed across the catalyst. if this happens with my LiH reaction that is fine.
but i dont want my reaction to superheat itself to above about 200C. i want to stay as close to the bare minimum total energy as possible.
|
|
|
5Qr25Pla
Harmless

Posts: 11
Registered: 18-2-2018
Member Is Offline
Mood: No Mood
|
|
in this post: http://www.sciencemadness.org/talk/viewthread.php?tid=3751 the user @HNO3 states that the "lithium 'wets' the steel crucible" and that it "forms a
hard, less reactive chunck that" he could not remove after melting. is there any reason my lithium should "weld" itself to my stainless steel to any
unremovable degree? i expect some to be stuck to the bottom. but my guess is that the majority should come up quite easily. (just considering how soft
lithium is)
... here's a pdf for anyone interested.
[Edited on 20-2-2018 by 5Qr25Pla]
Attachment: US2408748.pdf (844kB)
This file has been downloaded 496 times
|
|
|
5Qr25Pla
Harmless

Posts: 11
Registered: 18-2-2018
Member Is Offline
Mood: No Mood
|
|
it is not required but mixing the hydrogen with inert gas such as helium or argon (i will use helium) will reduce the heat produced by the apparantly
very exothermic reaction. this solves my problem of possible overheating of my reaction apparatus (stainless steel, but thin)
i will run the helium first through an inlet tube in my hydrogen production flask (so as to ensure homogeniety) and then heat my reaction chamber,
then after it is heated to 180-220C i will gradually introduce higher and higher levels of hydrogen until the reaction is either self sustaining or
"stable" enough to run to completion.
i am still looking for improvements though.
---------------------------------------------------------
improvements to be made:
further (potentially unnessecary) drying of the hydrogen/helium gases
removal of very minute levels of air that could/will be released from the water/aluminum reaction liquid (not a huge concern either as the water can
be pretreated to "remove/liberate" the air that has been dissolved in it before initiation"
------------------------------------------------------------
this entire apparatus is actually quite simple and cheap. and so far, all things required are easy to aquire. i hope to keep it that way. eventually i
hope to document the entire process in a very detailed manner so that others can produce hydrides. it is well understood that this process is
dangerous and is not for everyone, but there are those (myself included) that would love to benefit from this that can and will exercise safety and
caution.
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
http://gen.lib.rus.ec/book/index.php?md5=23E6EE80C42C2419E4B...
/CJ
[EDIT] - The link is now fixed. File too big for upload
[Edited on 22-2-2018 by Corrosive Joeseph]
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Non functioning link.
Copyrighted work by a member.
Book costs about US $170 to buy.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Everytime I see Magpie's sig, "The single most important condition for a successful synthesis is good mixing - Nicodem", I think of len1's disgraceful
failure and the subsequent arrogant attacks on Pok, and others, in the potassium sticky thread... I know you miss him, Mag, but I don't. 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, len1 really messed up there and got egg on his face. He owes Pok an apology. Nonetheless, his book is wonderful. There's nothing else like it
for the home chemist. Also, his contributions to Prepublication are magnificent.
He can be quite testy, however.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
I agree, his contributions are more than most. But in the spirit of this forum I see YOU as a much more valuable contributor. Plus, he got what he
wanted from this forum and still collects the royalties. Also, he got many eggs on his face, that was just the one that I am always reminded of when I
see your sig.
He never got "experimenter of the year"!
Edit: Also, he did apologize to Pok, it went something like, "I am still right for making the assumption I made, and you were wrong for not giving
enough clear, scientific data..." Even though pok clearly outlined his procedure and said, "Do it like this and see what happens..." When he followed
Pok's procedure, Potassium.
[Edited on 2-22-2018 by happyfooddance]
[Edited on 2-22-2018 by happyfooddance]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
He said here he got a flat fee and isn't worried about copyright that isn't his. The link works without the s on the http.
The lowest temperature reactions seem to be not simple and straightforward. The Chinese make an interesting statement about temperatures around the
mp.: "The hydrogenation rate increased with the raise of temperature at the beginning and reached a peak value at about 190C. Then the rate decreased
and almost no hydrogen absorption occurred in the range of 280-480C. The hydrogenation rate increased again when the temperature was higher than 500C
and achieved a maximum at about 665C. It was found that the optimal temperature range for hydrogenation of lithium was between 665 to 700C and with
the pressure ranged from 50 to 55 kPa."
What happens when one proceeds with Li dispersion in mineral oil under NaH synthesis conditions?
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
The link is now fixed.
I am a great fan of Len1's work, but as far as I am aware, he has NO rights to that book. They were all signed over to the publisher.
It was a labour of love and now we have it.
(And so we should, there are even a cluster of chapters in PrePub)
Also,there are a number of threads here about the book and there already is a link in References forum.
Enjoy

/CJ
[EDIT] - Post above was posted while I was writing this
[EDIT2] - Stupid grammar
[Edited on 22-2-2018 by Corrosive Joeseph]
[Edited on 22-2-2018 by Corrosive Joeseph]
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
I might just be really slow, but I have spent a good bit of time and still haven't been able to see even a single page of that book. I am sure it is
good, because you say so!
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Corrosive Joeseph  |
I am a great fan of Len1's work, but as far as I am aware, he has NO rights to that book. They were all signed over to the publisher.
It was a labour of love and now we have it.
(And so we should, there are even a cluster of chapters in PrePub)
Also,there are a number of threads here about the book and there already is a link in References forum.
|
I do appreciate len1's work. I have followed this forum for years, I learned chemistry from this forum! I didn't know that his book was accessible
here, I had only seen it "advertised" here. I don't mean to deprecate a long-time and valuable member, my apologies.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Somehow I missed whatever controversy happened. Before I was a moderator?
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
I am sure I was making a mountain out of a mole-hill. Len1 (and2) was a great contributor and his book is indeed excellent.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Pok showed us how to make potassium from KOH and a tertiary alcohol, IIRC. Len1 claimed Pok's work was bogus, when it clearly was not: We were shown
pictures of big balls of K. So, a protracted argument ensued. Pok, of course, was right. Len1 bowed out of the argument. Pathetic for a man of his
accomplishments and high stature. I guess we all get one mistake. His fault was in not admitting this graciously.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
2 |