| Pages:
1
2
3 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Diethyl Sulfate
December 7, 2017
A. Introduction
This preparation follows the method of Lynn and Shoemaker (ref1).
2C2H5OH + H2SO4 --→ (C2H5)2SO4 + 2H2O
Na2SO4 is added to the pot as a dehydrating agent.
B. Reagents
90g anhydrous Na2SO4
50g 95% ethanol
104.5g conc sulfuric acid
Na2CO3
C. Equipment
2-neck 1-L rbf (the “pot”)
distillation tapered glassware
hotplate
thermocouple (or suitable thermometer)
vacuum pump (or aspirator)
p-e funnel
oil bath
separatory funnel
D. Procedure
a. Reaction/Distillation
Caution: Diethyl sulfate is reputed to be poisonous when vapors are inhaled or the liquid is absorbed through the skin. Provide
adequate ventilation and wear gloves.
1. Setup the equipment for simple distillation using the 1-L rbf as pot.
2. Add 90g anhydrous Na2SO4 to the pot.
3. Pour 50g of the 95% ethanol in a beaker and cool in an ice bath.
4. Slowly add 104.5g of conc H2SO4 to the ethanol while cooling in the ice bath.
5. To the side neck of the pot attach the p-e funnel.
6. Pour the ethanol/acid mix into the p-e funnel.
7. In the center neck of the pot affix the thermocouple, or thermometer (See Note 1 below.)
8. Place the apparatus under full vacuum (≤ 45 mmHg).
9. Heat the oil bath to about 180°C. The goal is to bring the pot to 155°-165°C. (See Note 2 below.)
10. Add the ethanol/acid mix at 120-150 drops/minute. Distillate will collect at 2-3 drops/s.
11. Add all of the ethanol/acid dropwise. Distill until no more distillate accumulates.

Photo 1: equipment set-up

Photo 2: utilities
b. Separating, Washing, & Drying
1. Separate the distillate using a separatory funnel into diethyl sulfate and ethanol. The density of diethyl sulfate is 1.17 so it will be the
bottom layer.
2. Wash the diethyl sulfate with dilute Na2CO3.
3. Separate and wash the diethyl sulfate twice with water.
4. Separate, saving the diethyl sulfate and discarding the wash water. See note 3.
5. Place the ~30 mL of diethyl sulfate in a flask and add a scoop of anhydrous Na2SO4. Cap and set aside to dry.
6. Filter the diethyl sulfate and weigh for yield.
E. Results
My yield was 32.7g. Lynn & Shoemaker obtained 32.6g. %yield on ethanol is 41.1%.
F. Discussion
My first attempt to make diethyl sulfate was a miserable failure (see above, in a separate thread) due to inadequate equipment. More importantly, I
may have mixed up the diethyl sulfate layer with the dilute Na2CO3 layer during the washing step.
G. References
1. “The Laboratory Preparation of Diethyl Sulfate,” by EV Lynn & HA Shoemaker, January 3, 1924,
[Contribution from the Research Laboratory, Eastman Kodak Co, No. 199], Seattle, WA. See photos of paper here:
Notes
1. If a thermometer is used assure that it can be read in the 155°-165°C range.
2. My pot temperature never came up to 155°C until the very end of the distillation. This was due to clumping of the Na2SO4.
3. Distinguishing the diethyl sulfate layer from the dilute Na2CO3 layer can be tricky. Use the drop sink/float test. Density of diethyl sulfate is
1.17.
Comments, questions, and suggestions are welcomed.
[Edited on 7-12-2017 by Magpie]
[Edited on 7-12-2017 by Magpie]
 
[Edited on 7-12-2017 by Magpie]
[Edited on 7-12-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nice work Magpie. If you have the paper perhaps you could attach it to your post for convenience (although I think I am familiar with it). What is
your %th yield?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by DJF90  | | Nice work Magpie. If you have the paper perhaps you could attach it to your post for convenience (although I think I am familiar with it). What is
your %th yield? |
Thanks, Dan. I added the paper and the %yield to the original post on edit. I don't have a scanner. Hope you can read the paper.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Magpie, the reference for the paper is JACS 1924 46 (4) pp. 999 to 1001. I've attached it as a .pdf for
convenience.
Attachment: ja01669a023.pdf (65kB)
This file has been downloaded 1161 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Nice write up and very important for OTC preparation of sulfuric ether (diethyl ether)  
Just some side notes...
Side products coud be:
==> CH3-CH2-O-SO2-OH thus a partial sulfuric ester
==> CH2=CH2 (ethene) and H2SO4/H2O if the heating is slightly too high.
[Edited on 8-12-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by PHILOU Zrealone  | Nice write up and very important for OTC preparation of sulfuric ether (diethyl ether)  
Just some side notes...
Side products coud be:
==> CH3-CH2-O-SO2-OH thus a partial sulfuric ester
==> CH2=CH2 (ethene) and H2SO4/H2O if the heating is slightly too high.
[Edited on 8-12-2017 by PHILOU Zrealone] |
Thank you PHILOU. In review I feel that I did not know my true temperature because the Na2SO4 clumped around the thermometer bulb. When I get my
stepper motor mixer operating I will repeat this synthesis. I have Ba(OH)2 on order and will also do an assay of the product. I urge caution to
others who may wish to repeat this synthesis until I can report my assay results.
Do you happen to know the temperature range in which CH3-CH2-O-SO2-OH is produced?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
kmno4
International Hazard
    
Posts: 1503
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Na2SO4 is added to the pot as a dehydrating agent. |
Of course, Na2SO4 cannot serve as dehydrating agent in this case, because all its hydrates are stable only below 40 C.
It must be a component in this reaction, generating (in situ) some salts.
This procedure works, but not because Na2SO4 can dehydrate anything under given conditions.
Edit:
Literature reseach gives interesting information about given reaction, it is said that hydrogen ethyl sulfate reacts with Na2SO4 under vacuum and
heating, giving diethyl sulfate and double salt Na2SO4•NaHSO4.
So indeed, it has nothing to do with dehydration 
[Edited on 27-12-2017 by kmno4]
Слава Україні !
Героям слава !
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
On December 7, 2017 I attempted to make phenetol using the procedure in Brewster. This method uses phenol and diethyl sulfate. The synthesis was a
failure, casting doubt on whether or not I had actually made diethyl sulfate.
I was hoping I could find the error and make a correction in my procedure for diethyl sulfate but I have not had a chance to get to this in the
alloted time of one month.
Therefore, I will pursue this further when I get my overhead stirrer repaired, soon I hope.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
The smell is pretty tell-tale... Some say peppermint, I say candy shop... But it is hard to mistake. You didn't re-distill it, did you?
Edit: I know it's a known occupational carcinogen and one shouldn't go around breathing large amounts of it, but does one occasionally produce small
amounts of it when making other things, such as ether for example. I don't imagine it is as bad a thing as some people fear, like benzene in my
opinion. I try to breathe as little as possible, but somewhat enjoy it.
[Edited on 1-13-2018 by happyfooddance]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I did not redistill it. I have an excellent sense of smell and smelled only a trace of peppermint.
The synthesis of phenetole was a failure. I will try to make the DS again.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
I'm thinking addition of a Dean-Stark type apparatus here could improve yield, by returning the unreacted ethanol to the boiling flask, and allowing
continuous removal of the product as it condenses.
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PirateDocBrown  | | I'm thinking addition of a Dean-Stark type apparatus here could improve yield, by returning the unreacted ethanol to the boiling flask, and allowing
continuous removal of the product as it condenses. |
It would be hard or maybe impossible to keep the ethanol condensing and not the product, as the b.p. of ethanol is about 120°C below that of diethyl
sulfate.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Diethyl Sulfate (DS) 9/6/18
As before this prep followed the method of Lynn & Shoemaker (Kodak, 1924). 92.6g of anhydrous Na2SO4 was placed in a 2-neck 1000ml RBF located in
a silicone oil bath. The bath was heated sufficiently high (180° - 230°C) to keep the salt at 155°- 165°C. A mixture of 63ml of Everclear (95%
ethanol) and 57ml of 98% H2SO4 was added at 2d/s. A vacuum distillate of ethanol/diethyl sulfate was collected in a receiver flask. It smelled
faintly of peppermint.
The two phases were separated using a 125ml separatory funnel. The DS was washed with dilute aqueous Na2CO3, then twice w/water. Anhydrous Na2SO4
was added to the DS to dry it. The filtered DS weighed 11.4g. Lynn and Shoemaker obtained 32.6g for a 41.1% yield.
Questions, comments, and suggestions are welcomed.
 
More on next post
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
continuation of last post. Toggle picture to turn.
 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Hello Magpie,
Congrats for your synth and setup!
The low yield bothers me a lot though. You usually do much better. :-) (Just pulling your leg, you actually have much more diethylsulfate than me.)
Anyway, don't you think that preheating the dehydrating salt to that high temp caused a lot of ethanol to "flash distill" at the moment of dripping in
instead of going into reaction?
This particular synth is a hard one obviously. You either make ethylene around 130 C or diethylether around 160 C. If you are lucky you might end up
with some diethylsulfate too. :-) The great guys at Orgsyn were probably very proud of their 41%.
You have so many labware gadgets and such. Would it be possible to distill the "product" into a Dean-Stark - like something and recirculate the upper
layer continuously? (I know there is vacuum so a proper Dean-Stark may not work.)
Maybe a catalyst of some sort? As I read Al-sulfate promotes ethylene formation instead of diethylether. (Or vica-versa, I'm not sure.) Maybe another
inorganic sulfate would prefer diethylsulfate. Just thinking.
[Edited on 8-9-2018 by Pumukli]
As KMnO4 pointed out, Na2SO4 is a catalyst and not simply a dehydrating agent. So maybe another salt may work better.
Someone already suggested the Dean-Stark too. It seems I could not add any original ideas in this case.
[Edited on 8-9-2018 by Pumukli]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you Pumulki. After 16 years of collecting I do have all the gadgets I want. Give me a sketch of how you would set up a Dean Stark recycle of
ethanol to the pot. (ethyl)2SO4 density is 1.18.
I, too, am very disappointed in the yield. Why do the boys at OrgSyn always get such good yields? Actually this is a Kodak procedure, however.
Thanks for your thoughtful suggestions.
I'm going to do this synthesis again today. I will be paying especial attention to the salt temperature.
I had to troubleshoot my thermocouple. It was the damn wire. It just reads 2°C high now. Using a thermometer would be impractical due to the
fogging in the distillation adapter. If I had another TC I could control the oil bath temperature better using a PID controller. The drip rate is
also hard to control.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Incorporating the changes just discussed the run today was made with a revised apparatus. A peristaltic pump was set up to recycle unused ethanol and
PID control was used on the oil bath temperature. Also the long 24/40 condenser was used along with a 3-neck 1000ml RBF.

This run was aborted because no apparent diethyl sulfate (DS) was being made. This is attributed to the salt temperature being too high (~180°C).
Also the pump did not work as it needed to be primed first. I had to use a thermometer rather than a TC and could not see the salt temperature due to
fogging in the distillation adapter.
Another run will be made once I have:
1. Obtained a longer TC
2. Verified the pump is working.
[Edited on 8-9-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Very interesting setup, Magpie. I was also wondering about the yield. Is it possible that your vacuum is too strong? I'm not exactly sure what the
"filter pump" they used in the article was, but I had thought that a water aspirator was usually used for making diethyl sulfate.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, if the use of the peristaltic pump proves out it could be quite useful. I paid $150 for a glassblower to make a separator that could have been
replaced with this pump.
In my notes the vacuum specified is ≤45mmHg.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
If I'm reading the manometer correctly, it says 4 mm Hg. That could cause the reaction temperature to be a lot lower than if it were 20-30 mm Hg. I've
never done this, of course, but if the reaction is finicky, and I have every reason to think it would be, I would think that applying a vacuum of less
than 10% of what your notes specified might make a big difference.
[Edited on 9-9-2018 by JJay]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
My notes say "less than or equal to" 45mmHg. I think my inability to control the salt temperature using the thermometer is the culprit. I have an
18"TC on order.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That seems reasonable. It could be hard to monitor the rate of the reaction as well.
This chart is from "On the Vapor Pressure of Sulfuric Acid" by Ayers et al. The vapor pressure of 98% sulfuric acid is about 1.3 mm Hg at 180 c and
closer to .4 mm Hg at 160 C. I don't think it's a huge problem, but I don't think it's insignificant.
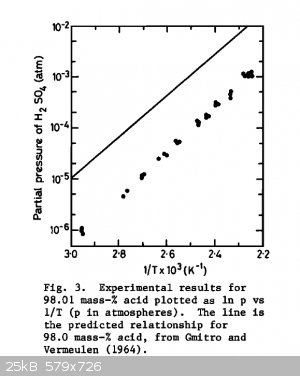
(Oh and the initial reaction between ethanol and sulfuric acid approaches equilibrium in about 10 minutes at 70 C and in a few hours at room
temperature. Source: https://cdn-pubs.acs.org/doi/10.1021/ja02248a014)
[Edited on 9-9-2018 by JJay]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I tried this synthesis again keeping the temperature of the salt on the low side. I got not diethyl sulfate (DS), only alcohol. I am going to give
up on this synthesis using ethanol and sulfuric acid.
I will now attempt to produce ethylene in usable quantity using ethanol and aluminum oxide in a tube furnace. The ethylene can then be reacted with
sulfuric acid to form the DS.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by kmno4  | | it is said that hydrogen ethyl sulfate reacts with Na2SO4 under vacuum and heating, giving diethyl sulfate and double salt Na2SO4•NaHSO4
|
I have to wonder if the overall reaction isn't Na2SO4 + 2EtHSO4 -> 2NaHSO4 + Et2SO4
as Kuh (US1411215 etc) said
Na2SO4 + EtHSO4 -> NaHSO4 + NaEtSO4
EtHSO4 + NaEtSO4 -> Et2SO4 + NaHSO4
[Edited on 6-10-2018 by S.C. Wack]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's the data and results from the 3 Et2SO4 runs I have made:
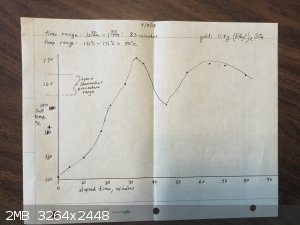 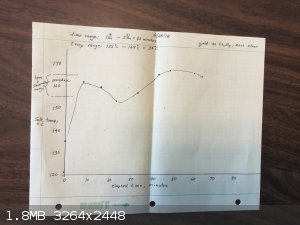 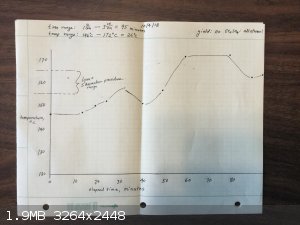
There does not seem to be any significant difference between the runs in comparing the temperatures to the Lynn & Shoemaker recommended range.
I tested the density of the Et2SO4 I made on the first run: it is 1.15 g/ml. Book value is 1.18.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
2
3 |