Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
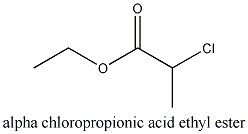
alpha-chloropropionic acid ethyl ester
An interesting compound.
How to prepare it?
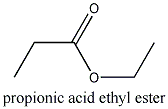
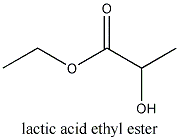
The ethyl ester of propionic acid or lactic acid can be prepared by venting dry HCl-gas into a mixture of ethylalcohol and the acid.
alpha-chlorination of propionic acid:
- with PCl3 (not easy)
- with thionylchloride (?)
- with PCl3/TCCA (see here)
alpha chlorination of lactic acid:
- with PCl3 (?)
- with thionylchloride (95% yield)
- with PCl3/TCCA (?)
- with zinc/HCl (?)
I am convinced that lactic acid (or it´s ester) is a better starting compound for this preparation. With propionic acid (and it´s esters) problems
are common and I read articles where researchers were less than enthusiastic about preparations which were told to work by their predecessors.
The alcohol group of the lactic acid seems to be a better point of attack.
For home experimenters only two methods seem feasible:
The PCl3/TCCA method which needs only catalytic amounts of PCl3 which can be prepared in situ - even some red phosphorus scraped from matchboxes will
do the trick, and the Zn/HCl method.
Will the Zn/HCl work? No doubt, it *will* chlorinate the -OH group - but will it leave the rest alone? Thats my question.
Some pictures for a better understanding:
Thats what we want:
All ideas, critics suggestions, illusions & delusions, flashes of genius and subgenius are welcome!
/ORG
[Edited on 29-5-2005 by Organikum]
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Lactic acid is OTC at reasonable prices, have a look here
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
"Will the Zn/HCl work? No doubt, it *will* chlorinate the -OH group - "
I am curious as to your confidence about this. I attempted this with lactic acid (excess HCl, spatula of reagent grade zinc dust, and 4 hour reflux)
without success. The only potential problem I could see was the inclusion of calcium ions in the solution (since the lactic acid was coming from the
HCl acidification of Calcium Lactate.).
I dont suppose you have any digital references readily available? I do remember reading a reference similiar to this but using isopropyl alcohol
instead of lactic acid. Having a reference that implicitly states that lactic acid works would help me to identify the mistakes I am making.
Also, and maybe I am missing something obvious here, why dont you want to esterize after chlorination? I would think it would be a lot easier, and
the reaction conditions you state for esterization dont strike me as something that is going to affect that chlorine much.
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
I agree that esterification after chlorination is preferred as the ester might hydrolize under the conditions.
The HCl/zinc-chloride chlorification is from the Gattermann (german). I will look it up later and post it. You might have had a not sufficient
concentration of HCl in the solution - the chlorination actually proceeds through some dubious zinc-chlor-acid and the formation of this requires a
very high concentration of HCl present. the addition of Zn as metal has already diminishes the HCl - did you use 37% HCl? Maybe venting gaseous HCl
into the solution would help to drive this forward. Pressure would as I believe.
/ORG
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Ah, I see. Should I attempt this synthesis again, I shall use a modified procedure. If the concentration of the HCl is of paramount importance, then
refluxing isnt a very good idea, as it reduces the acid content to about 20%. (Unless a pressurized vessel is used, and I like many other hobby
chemists do not possess the proper equipment for this.) Perhaps this is one of those reactions that is best "mix, seal, and forget for a
month", yes?
Also, I have a question I have wanted to ask for some time but kept forgetting to do so. Will lithium alkoxides (ie lithium methylate) serve as well
as sodium alkoxides in the Darzens glycidic ester condensation?
If anyone can answer this it would be most helpful.
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Didn't find any references for using lithium. Only some reactions where potassium was used instead of sodium, but I don't think that helps
much..
But I did find a cheap source for lactic acid (5€/l) and ordered few liters (should arrive next week) so I can try the Zn/HCl too to get some
comparable results.
Also, if only small amounts of PCl3 are needed I might give the PCl3/TCCA a go too.
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
100ml of 80% lactic acid was refluxed for 2h in an oil bath with 85ml 35% HCl and 1mol of ZnCl2. Mixture was initially golden yellow and clear but
turned dark while refluxing. Some gas was evolved for a short period. I guess it was just HCl. Should I now distill the mixture? I don't have a
vacuum source but CRC doesn't mention the 2-chloropropionic acid decomposing at its boiling point (185C). Everything else should come over before
the chloro-acid starts to distill (lactic acid decomposes in atmospheric distillation, but into what?).
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by IPN
100ml of 80% lactic acid was refluxed for 2h in an oil bath with 85ml 35% HCl and 1mol of ZnCl2. Mixture was initially golden yellow and clear but
turned dark while refluxing. Some gas was evolved for a short period. I guess it was just HCl. Should I now distill the mixture? I don't have a
vacuum source but CRC doesn't mention the 2-chloropropionic acid decomposing at its boiling point (185C). Everything else should come over before
the chloro-acid starts to distill (lactic acid decomposes in atmospheric distillation, but into what?). |
Hmm, one entire mole of ZnCl2? Thats a lot, I never would have guessed to try using that much.
As for recovering the product, I would recommend extraction with a chlorinated solvent, such as DCM or Chloroform. My understanding is that diethyl
ether will work as well. Im not sure, but I would guess that the 2-chloro-acid would distill over with the water. I suppose that measuring the
temperature of your distillate would help to make clear wether or not this happens.
I am also attempting this again, though by a decidely different route.
.5 mol of clacium lactate (1 mol lactate ion) was added to 250 ml of 37% HCl. In a seperate beaker containing 50mL of 37% HCl was dissolved a
"heaping spatula tip" (~2g) of zinc metal powder. After the evolution of hydrogen stopped, this 50mL was added to the 250mL flask.
Hydrogen chloride was then pumped in (formed by the action of sulfuric acid on NaCl), enough so that the solution ought to contain roughly ~44%. (Did
you know that an ice cold aqueous solution of HCl can contain up to 85% HCl?  ) )
The above flask was then sealed and has been sitting on a shelf for around 72 hours now. Tomorrow afternoon I intend to heat it up to around 60C for
about 6 hours before letting it cool down and attempting extraction with DCM.
BTW thanks for looking for info on the darzens, IPN. I have come to a similiar conclusion. My guess is that it does not work, as it is never
mentioned and the chemistry of lithium and sodium can be suprisingly different. Anyways, let me know how the chlorination works out. 
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Hmm, no luck
Well, my method as posted above, except that the solution was left in the flask for 120 hours (w/ 6h @ 60C), and was then extracted with 75mL x1 then
50mL x2 of DCM.
Distillation of the DCM gave...nothing. 
It should be noted that during this time no visible change of the solution was ever observed.
Oh well, back to the drawing board. I'm thinking that either the zinc chloride concentration isnt high enough or the calcium ion prevents the
zinc/HCl adduct from forming. IPN, Org, your thoughts?
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
I first filtered the tar looking liquid and then treated it with some activated carbon. That didn't clear it much..
Then I tried to extract it with chloroform but of cource I couldn't see the layer.. Well, with the help of a flashlight I found out that it had
emulsified  even though I carefully turned the s.funnel up and down taking care not
to shake it. So now I guess I just have to wait for it to separate. even though I carefully turned the s.funnel up and down taking care not
to shake it. So now I guess I just have to wait for it to separate.
NaturesNatrium, I think the problem is with the correct amount of Zn/HCl. 1 mol of ZnCl2 was certainly too much.. Maybe the reactants also need to be
quite dry?
EDIT:
Got nothing but tar and very dirty glassware after distilling off the chloroform. 
[Edited on 10.6.2005 by IPN]
[Edited on 11.6.2005 by IPN]
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
I tried the following. To the left over aqueous solution from above was added another half mol of lactate ion, 4 more grams of zinc (pre mixed with
30 mL of 37% HCl), and gassed with HCl to saturation at room temp. The solution was then refluxed for four hours. There was a discoloration, and the
formation of a small amount of insolubles. (And an evolution of a whole lot of HCl gas.) This was cooled, extracted with DCM (1x 75, 2x 50), and
the DCM dried over calcium chloride. The DCM was distilled to yield absolutely nothing. 
Hmm, I dont think I am going to try this method again unless some new information comes to light. Since I was told in a private communication that
calcium chloride has been used in place of zinc chloride in these reactions, I dont believe the calcium ion is the problem. I'm sure DCM is an
acceptable solvent for biphasic extraction of the alpha chloro compound. I just dont know what else to try.
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
The solution from above had been sitting around for a bit, and had discolored thoroughly to a bright yellow green. Extraction with 50mL of DCM
yielded nothing. The aqueous solution was discarded and the DCM recovered.
Hey Org, you got that article you said you might post? (Delay...searching....oh!)
Hey, I have the gatterman.  Here is what he had to say about it: Here is what he had to say about it:
Hydrochloric acid reacts with most difficulty, and it is, e.g., in the preparation
of methyl chloride and ethyl chloride, necessary to employ a
dehydrating agent — zinc chloride is the best — or with the alcohols of
high molecular weights, to heat in a closed vessel under pressure.
Well, obviously an aqueous solution isnt going to get the job done. A dehydrating agent, hmm. Ah! That's why calcium chloride might work, as
long as it is anhydrous. Back to the drawing board then. 
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Well, I moved on an interesting notion I had. My thoughts were that since a dehydration agent is involved with the HCl chlorination of lactic acid,
that there must be two possible pathways, perhaps both of which occur to some extent.
First, and what I originally assumed was true before thinking about it a bit, is that the H+ adds to the alpha position hydroxyl, giving water which
is easily replaced by a chlorine ion. In this scenario the dehydration agent is acting as a equilibrium shift device by absorbing the water as it is
formed.
The other possibility that occured to me is that the elimination of water actually comes first, hydroxyl from alpha and a hydrogen from beta, to form
acrylic acid and water. This is then immeadiatly attacked by a H+Cl- pair to add across the double bond, a markinikov addition which yields a
chlorine atom in the alpha position. Although according to Merck acrylic acid can polymerize in the presence of HCl, the fact that it is formed then
immeadiatly reacted would probably lead to very low polymerization if any.
I would also like to mention I looked into acrylic plastics as a source of acrylic acid directly, but it seems things are not simple. There are many
kinds of plastic that are labeled acrylic, which include compounds such as acrylonitrile, methacrylic acid, acrylamide, various esters of acrylic
acid, and co-polymerization agents such as styrene and 1,2-butadien. However I don't think sourcing acrylic acid itself (or interesting esters
such a methyl or ethyl) would be difficult for a person learned in hobbyist chemistry. The difficult part would be getting the acid to add across the
double bond without inducing polymerization.
Anyways, the thing I attempted was to take calcium lactate(.125mol), add a bit of water to make a paste, neutralize with aqueous HCl, and distill off
the excess water (~50mL). Then to the flask was added a heaping spatula of NaHSO4 as a dehydration catalyst. The mix was then heated quite warm on a
hotplate, until at last a distillate began to come over.
The temperature of the ditillate was quite consistant between 110 and 120C. The distillation was stopped after collecting about 25mL. The inside of
the reaction flask was covered in white salts (haven't cleaned it yet, please dont let it be polymerized acrylic!). The distillate product is a
clear liquid with a very odd odor. I really don't have words that can accurately depict the smell, although I can say I don't find it
pleasant. The liquid is thoroughly miscible with water, and passes the permanganate double bond test, but fails (I think) the bromine test. I say
"I think" because I have never done the bromine test before. The red quickly goes to yellow in this case, when a few drops are added. Of
interest is the fact that the product doesnt appear miscible with DCM. Also, its freezing point is below -10C.
So, based on this, I don't think I have acrylic acid, but it begs the question, what do I have in my flask? Any takers?
Oh, and before someone mentions it, I have to admit I am absolutely horrible at all forms of chromatography which dont involve a machine doing all the
work (which is not available to me right now).
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Addition of HCl on acrylic acid can only yield 3-chloro-propionic acid (Cl-CH2-CH2-COOH) and not 2-chloro-propionic acid which you want. The
nucleophyle (chloride) adds to the most electrophylic side of the double bond and the proton on the most nucleophylic side. It is always as simple as
that (unless the mechanism is radical). In a case like this, where the double bond is polarized trough resonance by an electronwithdrawing group, the
reaction is called a Michael addition or more generaly a 1,4-addition.
Distilling calcium lactate from a mixture of hydrochloric acid and sodium hydrogensulphate can only give you a water solution of lactic acid and HCl.
Obviously such a mixture reduces/discolors KMnO4 since lactic acid gets easily oxidized to pyruvic acid and even if it was not so, HCl also discolors
KMnO4 by being oxidized to Cl2. Such a test does not prove the presence of double bonds.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Hmm, damn, I guess I need to hit the books again. I knew it was too simple to be true. 
Of course, I didn't think about the fact that 2NaHSO4 + CaCl2 -> CaSO4 + NaCl + HCl is a possibility. It would also explain the high pH and
the reduction of the permanganate ion. It doesnt necessarily explain the unusual distillation temp, unless that happens to be the temp at which the
equilibrium for the above reaction is shifted to the right.
Wait, I remember reading in the Merck that Lactic acid is volatile in superheated steam. If the salt mixture raised the bp of the water high enough,
then perhaps I did end up with aqueous lactic acid in the recieving flask.
I find it interesting that in literature one only sees KHSO4 as a dehydration catalyst but never NaHSO4. Anyone know why this is?
Thanks for the info Nicodem, much appreciated.
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
<b>Organikum:</b> What do you think about Alanine as starting-material? You can substitute NH2 with Cl to get the
<i>α</i>-Chloropropionic acid:
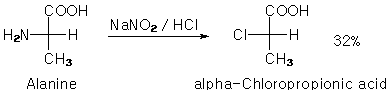
I've got a short description for this from <i>Conrad Weygand / Gustav Hilgetag</i> "Organisch-chemische Experimentierkunst"
(1970). But there are for sure more actual procedures out there.
~Mephisto
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Could lactic acid be dehydrated first (I have it as an 80% aqueous solution) with anhydrous CaCl2 and then extracted from the paste with DCM or
toluene?
The DCM(or toluene)/lactic acid could then be mixed with some more anhydrous CaCl2 and dry HCl bubbled in. The CaCl2 should absorb the water formed in
the reaction. I'll try this out soon.
|
|
|
liwenbinyk
Harmless

Posts: 3
Registered: 6-12-2005
Member Is Offline
Mood: No Mood
|
|
CaCl2 will dissolve in water and lactic acid very well, so extraction will be very difficult.
why not dist the aqueous lactic acid, boiling point of lactic acid is higher.
|
|
|