chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
Synthesis of colorful quantum dots from table sugar
So a while back when I was first learning about hydrothermal synthesis, I was fascinated by one of the main side products that show up almost every
time a carbon source is present in the reaction; quantum dots. Thing is, no matter what carbon source you use you almost always get blue quantum dots.
I wanted to make other colors so I dug into the literature and there was only 1 paper I could find detailing how other colors could be made starting
from carbohydrates. Unfortunately, it was the most poorly written paper I'd ever read AND it was publish in nature.
So I spent a few days tinkering with the protocol until I managed to make a few different colors. All things considered it's actually really straight
forward and only requires sucrose as a starting material. For reds and yellows you need phosphoric acid, and for blues and greens you need
hydrochloric acid.
I made a video detailing my experiments, results and the poorly written mess that is the paper:
https://www.youtube.com/watch?v=W_Xa-m5zMYI
[Edited on 5-10-2017 by chironex]
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Fantastic work! I think this is an under appreciated field of research and worthy of tinkering and experimenting. Keep up your postings on the topic.
I'm feeling inspired by it.
|
|
|
morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
Are you sure these are quantum dots? Acidic catalysed decomposition of sugars lead to fluorescent organic compounds which can be separated to nonpolar
solvent.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Not being a smart arse but, are your prelim amounts correct for sucrose? you mention 1.5g & then 0.7g twice in the first part of the video. I am a
noob so just wanted to check that the there is indeed two lots of 0.7g sucrose used as per vid.
thanks
edit
sorry my bad, its late and i misunderstood!! so it is 0.7g twice. Appologise again, i should learn to pay more attention 
[Edited on 7-10-2017 by NEMO-Chemistry]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Trying to redeem myself for the above nonsense i posted....
Would it be more likely they used Glucose instead of sucrose? Sucrose according to wiki dosnt have a melt point, it decomposes which is kind of what
we see in the video. Glucose has a melting point of around 146C, so would it be worth trying Glucose instead?
Edit 2
Not having much of a clue what i am talking about, but i got curious and found this.
Does this help at all?
[Edited on 7-10-2017 by NEMO-Chemistry]
Attachment: wang2011.pdf (410kB)
This file has been downloaded 422 times
|
|
|
chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
Glad you enjoyed 
Far as I know they are quantum dots. Through all my time doing this I've been able to get a pretty decent spread of colors so it's unlikely to be
simple fluorescent molecules. If I had access to a TEM I could double check but I don't atm.
As to the glucose vs sucrose, it really shouldn't matter. At least hydrothermally I've gotten quantum dots using everything from orange juice to
gelatin to tannic acid. Obviously things aren't as controlled in those circumstances, but the fact that the product is reasonably consistent is
telling. If you were really serious about this though, glucose would be a good thing to start with.
In all cases the carbon source gets degraded and converted into hydroxymethylfurfural which then goes on to assemble into the larger structures and
eventually the quantum dots. The process is a lot more complex than that, but that's the short version.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
The paper i posted seems to give another or at least a similar method.
|
|
|
chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
Ya there's lots of ways to make them. They almost all revolve around "put carbon source in water" "heat" "maybe mess with the pH". The microwave
approach is nice cause it's fast and you can get some colors I've only seen with that method like violet. In my mind it all falls under the
"hydrothermal" or "solvothermal" umbrella though there's some distinct differences in what's going on chemically in each which allows you to tune the
final particles properties. Solvothermal, so using solvents other than water, typically makes smaller rounder particles, but again that'll vary wildly
based on the conditions and starting materials.
|
|
|
Jimmytheone
unable to post anything

Posts: 7
Registered: 6-10-2017
Member Is Offline
Mood: No Mood
|
|
What are quantum dots? Are these like micro dots?
|
|
|
morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
At least with green i would try extraction with non polar solvent to confirm it´s really not just some organic compound.
|
|
|
morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
Yeah I tried it and it seems that it´s really quantum dots
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I am not good at explaining stuff, and quantum dots are a bit special, however wikipedia seems to have some good info thats not too hard to
understand, so rather than me mess up the explanation, have a look at this...... HTH
https://en.wikipedia.org/wiki/Quantum_dot
|
|
|
chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
Well that answers that then. Cool! What color did you end up making?
I still wish I could figure out how to make red. That's been the most elusive by far.
|
|
|
Zosimos
Harmless

Posts: 1
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
I used the HCl and NaOH methods and got green each time. I also used a few mls of 10 mM copper sulfate solution and got green. More interesting to me,
i used only 3g of sucrose and 10 ml of water and boiled it in a covered flask for an hour, occasionally adding water, and got a soft lime green glow
from it. This concept has definitely hooked my curiosity 
|
|
|
chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
In some earlier runs I noticed weird results if metals were added. I made a vibrant yellow batch that contained iron and a very green batch that
contained copper. I wonder if the dots act as a template and end up coated with metal oxides. If they're still in the size range after that they would
also behave strangely. Either that or the metals dope the carbon. Eitherway, I feel there's a lot of interesting potential with these.
I tried shooting various lasers through various batches, I think these could almost be used for a dye laser. Would be very interesting if it was
possible. Then you could adjust the laser's output wavelength by adjusting the size of the particles. And I doubt they'd suffer from the same
degradation issues that the various laser dyes do.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Hi. Would you mind telling us what kind of UV light source you are using ?
A quick experiment with polyethylene glycol (brake fluid) and fructose in the microwave for 5 minutes produced 'something', mostly a brown tar and an
unusual smell.
When illuminated with a cheap UV flashlight it looks pretty much the same colour as raw PEG.
|
|
|
chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
I've had that problem before. You need a shorter uv wavelength and the really cheap uv flashlights wont work. So either your reaction didn't work, or
your wavelength is too long. 365-390nm is ideal. Also the different colors fluoresce differently at different wavelengths. Blue glows better at 360,
yellow glows better at 390
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Great ! Thanks for the info.
New UV lighting is in the post 
|
|
|
CRUSTY
Hazard to Others
  
Posts: 139
Registered: 5-6-2016
Location: Nearby
Member Is Offline
Mood: High-Order
|
|
Alright, so I just tested the microwave-assisted synthesis detailed in this paper. I'm not sure what to think of the results.
I conducted three trials, the first being at 930 watts microwave power, the second two being at around 500 watts. 10 g sucrose was used as the carbon
source for each trial. The first two used 2 grams NaCl added to 75 mL water, whereas the third used CaCl2 instead. I thought I
would stop them after slight yellowing of the solution occurred, but the higher power in trial 1 led me to overshoot and caramelize much of the
sucrose.
When exposed to 100 mW laser light at ~405 nm (although I think my laser wanders a little shorter than that), a greenish fluorescence was observed for
batches 1 and 2. Batch 3 showed fluorescence that was slightly green but appeared to be more towards blue.
This was interesting and all, but I do have one concern, and that's about diacetyl. Diacetyl is produced in the caramelization of sugar, and has a
bright green fluorescence under violet to near-UV light. I know diacetyl is present in trial 1, but I'm unsure if it is present in the latter two
trials. I certainly couldn't smell it in the latter two. I want to believe that I have made quantum dots, but the potential presence of diacetyl make
me question if I really have. Any input is greatly appreciated, because I'm not sure what to decide or how to test it if need be.
I'd love to write more about this, and I probably will, but I'm exhausted. I'll see if I can do the full spectrophotometer fluorescence spectra on the
samples tomorrow.
|
|
|
CRUSTY
Hazard to Others
  
Posts: 139
Registered: 5-6-2016
Location: Nearby
Member Is Offline
Mood: High-Order
|
|
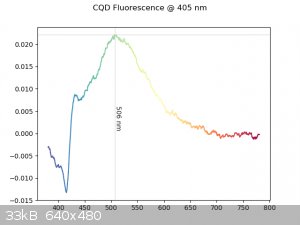
In case anyone is interested, I took the raw fluorescence spectrum at 405 nm of a batch prepared using aqueous sucrose and around 20% by volume dilute
aqueous ammonia (I didn't feel like determining the concentration of the solution I used at the time), using a reaction vessel very similar to what
chironex used. The vessel was heated at 175 °C for 1 hour. The spectrophotometer I used doesn't have a filled-cuvette calibration function, so I have
to take the transmittance intensity spectrum and compensate for it after taking the actual fluorescence spectrum. Unfortunately, I didn't have the
time to do this today, so the spectrum below is very clearly unfiltered, but the fluorescence peak is still visible, just extremely noisy. Nothing too
useful, but nonetheless interesting.
|
|
|