DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
Detonation of Methanol And Aluminium Mixtures
I read a few articles on the subject of hazardous chemical reactions and a few of them mentioned mixtures of powdered aluminium or magnesium and
methanol are capable of detonation with power exceeding some military explosives.
Has anyone had any experiences with these mixtures? I'd imagine you would need a big booster and i would be worried about the corrosion of the
aluminium.
Methanol is corrosive for some metals, including aluminum. Although a weak acid, it attacks the oxide coating that normally protects the aluminum from
corrosion:
6 CH3OH + Al2O3 → 2 Al(OCH3)3 + 3 H2O
The aluminum methoxide produced is soluble in methanol. As a result, the aluminum surface is cleaned of its oxide coating and then is readily oxidized
by some dissolved oxygen. Also, the methanol can act as an oxidizer:
6 CH3OH + 2 Al → 2 Al(OCH3)3 + 3 H2
This reciprocal process effectively fuels corrosion until either the metal is eaten away or the concentration of CH3OH becomes negligible.
|
|
|
Chisholm
Hazard to Self
 
Posts: 62
Registered: 2-4-2017
Member Is Offline
Mood: No Mood
|
|
The particle size of the metal would need to be insanely small, bordering on or inside the range of pyrophoricity. It may be possible for a strong
shockwave to initiate a reaction that (if run to completion) releases aluminum hydroxide, elemental carbon, and hydrogen gas, but there really isn't
anything special about methanol besides its cheapness. Glycerol (especially if thickened with a percentage of erythritol or dextrin) would surpass
methanol due to the higher density, larger percent oxygen per unit mass, and more stable air bubbles for adiabatic compression and hot-spot formation.
Of course, a glycerol-based mixture would require a sealed container or other method to exclude moisture.
[Edited on 7-29-2017 by Chisholm]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Chisholm  | The particle size of the metal would need to be insanely small, bordering on or inside the range of pyrophoricity. It may be possible for a strong
shockwave to initiate a reaction that (if run to completion) releases aluminum hydroxide, elemental carbon, and hydrogen gas, but there really isn't
anything special about methanol besides its cheapness. Glycerol (especially if thickened with a percentage of erythritol or dextrin) would surpass
methanol due to the higher density, larger percent oxygen per unit mass, and more stable air bubbles for adiabatic compression and hot-spot formation.
Of course, a glycerol-based mixture would require a sealed container or other method to exclude moisture.
|
It works onto the principle of high affinity of some metals for oxygen...a kind of organic thermite based reaction.
If the mix is intimate enough ==> ultrafine metals then the reaction can take the oxygen and reduce its carrier to lower oxydation states...
3CH3OH + 2Al ---> Al2O3 + 3 CH4 (or C2H6/C2H4/H2/C/C2H2/...equivalent mixes)
Same could be acheived with Mg.
Even Zn is known oxygen scavenger in rude organic chemistry..by heating with phenol for example one gets benzene/diphenyl (reduced form of phenol) and
ZnO.
Theorically Phosphor could also be a potential candidate...but way too dangerous and toxic to handle...
As you explained...to get enough heat per unit of volume...of course increasing the amount of available oxygen per unit of volume would be an
asset...just like a lower volatility.
One may even think to suggar/Al or even to better alternatives ==> unconventional explosives...
Like dry oxalic acid or tartric acid:
HO2C-CHOH-CHOH-CO2H + 4 Al --> 2 Al2O3 + 4 C + 2 H2(g)
3 HO2C-CO2H + 8 Al --> 4 Al2O3 + 2 C + 3 H2(g)
Dryness is mandatory to avoid acid/metal reaction leading to Al tartrate or oxalate and H2(g) and heat...bad news for storage.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chisholm  | The particle size of the metal would need to be insanely small, bordering on or inside the range of pyrophoricity. It may be possible for a strong
shockwave to initiate a reaction that (if run to completion) releases aluminum hydroxide, elemental carbon, and hydrogen gas, but there really isn't
anything special about methanol besides its cheapness. Glycerol (especially if thickened with a percentage of erythritol or dextrin) would surpass
methanol due to the higher density, larger percent oxygen per unit mass, and more stable air bubbles for adiabatic compression and hot-spot formation.
Of course, a glycerol-based mixture would require a sealed container or other method to exclude moisture.
[Edited on 7-29-2017 by Chisholm] |
I'm not sure you need to exclude moisture.
Magnesium will burn in steam.
Since the reaction of Mg and H2O is exothermic, it's quite possible that a mixture of the two could be detonated.
It would, of course, be horribly unstable.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Al powder/H2O2 watergels are detonable.....
Adding some organic fuel may help for viscosity ==> suggar/erythritol/glycerol.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I remember reading that thumbing through some reference book/Hazardous Chemicals Desk Reference at my university library one day.
https://books.google.com/books?id=q-6-fCfskYMC&pg=PA849&...
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I have had no luck. I used AN, 35% H202 and GD Al
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
It strongly depends onto conditions:
==> How much did you tried?
==> Into what diameter?
==> Into what confinement recipient?
==> With what detonator and booster and quantity?
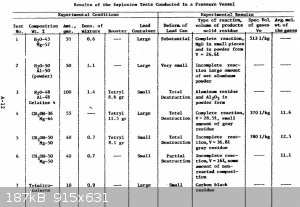
Into previously posted datas from Dr Manhatan you see that they used at least 50 grams with boosters of Tetryl arround 9gr...
Usually water slurries/gels require:
-high diameter (thus large quantities),
-good confinement (strong casing sometimes even rock drill hole itself),
-a very strong booster and detonator...
If made on site H2O2 tends to insert tiny bubbles that are desirable to increase sensitivity to initiation and reduce critical diameter of detonation
...a gelling agent tends to helps getting those bubbles and AN crystals/Al powder dispersed and slows down their segregation to the upper part (gas)
or bottom part (solids) of the recipient (thus reducing homogeneity/mixing and sensitivity)...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
I wonder if a composition of methanol/aluminium and some sort of gelling agent would work. You could possible sensitize the mixture with the addition
of Nitromethane.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by DrManhattan  | | I wonder if a composition of methanol/aluminium and some sort of gelling agent would work. You could possible sensitize the mixture with the addition
of Nitromethane. |
Why bother with the methanol at all?
https://www.google.com/url?sa=t&source=web&rct=j&...
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
Nitromethane based explosives are well known, well documented, and in regular use today. Methanol based compositions however are not, which is why
they intrigue me and hence the reason for this thread. I dont think i have ever heard of an explosive comprised mainly of methanol or whether such a
mix is even possible.
|
|
|
Chisholm
Hazard to Self
 
Posts: 62
Registered: 2-4-2017
Member Is Offline
Mood: No Mood
|
|
I'm almost certain it is.
Idealized equation:
2 Al (s) + 3 CH3OH (l) = Al2O3 (s) + 3 CH4 (g)
Std enthalpy change of formation:
∆f H methanol: –238.4 kJ/mol
∆f H methane: –74.87 kJ/mol
∆f H ethane: –83.8 kJ/mol
∆f H aluminium hydroxide: −1277 kJ/mol
∆f H aluminum oxide: −1675.7 kJ/mol
Standard enthalpy of reaction (∆f H products – ∆f H reactants):
2 Al (s) + 3 CH3OH (l) = Al2O3 (s) + 3 CH4 (g)
((1 * -1675.7 kJ/mol) + (3 * -74.87 kJ/mol)) – (3 * -238.4 kJ/mol) = -1,185.11 kJ/mol
So, the ideal reaction that completely reduces the methanol to methane and oxidizes the aluminium to alumina is strongly exothermic.
However, partial reduction to ethane and oxidation to aluminium hydroxide is considerably more exothermic:
2 Al (s) + 6 CH3OH (l) = 2 Al(OH)3 (s) + 3 C2H6 (g)
((2 * -1277 kJ/mol) + (3 * -83.8 kJ/mol)) – (6 * -238.4 kJ/mol) = -1,375 kJ/mol
You obviously won't get an ideal reaction; The carbon fragments flying around and recombining will probably give a mix of methane and ethane, with
some ethylene, acetylene, benzene, and so on present in trace quantities. Also, some aluminium will be oxidized to alumina and some to the hydroxide.
2 Al (s) + 6 CH3OH (l) = 2 Al(OH)3 (s) + 3 C2H6 (g)
((2 * -1277 kJ/mol) + (3 * -83.8 kJ/mol)) – (6 * -238.4 kJ/mol) = -1,375 kJ/mol
Now, regarding the activation energy (and thus the critical energy fluence, and from that the required strength of the cap/booster and degree of
confinement), that depends entirely on the size of the aluminum particles and how long they have been in contact with the methanol.
Anhydrous methanol has the ability to corrode aluminium if the passivation layer is punctured, forming aluminium methoxide and hydrogen. I would
imagine that over time, this would decrease the efficacy of the mixture as an explosive.
If you are using something like German dark 3 micron aluminium powder and anhydrous methanol, I'd say a decent booster charge such as 0.5 grams of ETN
(sealed in a thin plastic container, obviously) should touch it off.
If you have access to aluminium nanoparticles, the composition might be cap-sensitive.
If you wanted a nontoxic, nonvolatile gel that operated on the same principles, perhaps 3 grams aluminium, 8 grams glycerol, and 4 grams erythritol
stirred into a grey paste and crammed into a PVC tube would work, with 0.2 grams ETN and an appropriate primary for initiation.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Would magnesium be any better than aluminum? Granted it too will form methoxide and more readily than aluminum.
As an aside, I once put a magnesium fire starter in methanol and with a little heat started the chunk of metal fizzing like an Alka-Seltzer with the
release of hydrogen. I had to polish the surface to start the reaction. The stuff that remained after the reaction has some powerful gelling action if
mixed with water, it would clog a drain.
|
|
|