edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
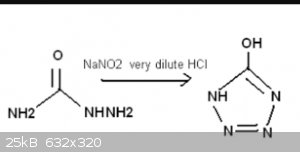
Semicarbazide ---> Sodium Azide NaN3 method
I was reading about semicarbazide and i found a method that uses it in the synthesis of sodium azide NaN3..
I need to make lead azide for my detonators, however I do not want to inhale hydrazine vapor by doing the most practical known method H2NNH2 • H2SO4
+ isopropyl Nitrite + NaOH ... I have everything necessary for make , but before I wanted someone's opinions .
Somebody alredy made for this way before ?????
Read the method here
9 grams of urea nitrate was added in small portions to 32 mL of ice cold (-3 C) concentrated sulfuric acid at such a rate that the temperature did not
exceed 5 C. Total time for addition was one-half hour, After which the mixture was poured on 75 grams of ice. The white precipitate was filtered,
washed with ice cold water, just sufficient to cover it, and air dried. Material obtained weighed 5.3 grams and melted with decomposition at 157-158
C.
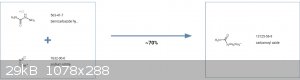
20g of nitrourea, 65g of zinc dust and 10 mL pure methanol (without water) are mixed in mortar and ground into a thick paste. A glass beaker is filled
with 12 ml of glacial acetic acid and 15 ml of pure methanol, then placed into an ice water bath. The cold nitrourea and zinc paste is added in small
portions with gentle stirring. The reaction will give off much heat, so the initial portions should be smaller, and added over longer intervals of
time, so the mixture does not overheat. The rate of addition should be adjusted to keep the temperature from rising too much, ideally around 10 degC.
Do not allow the temperature to rise to 35C. If the mixture becomes too thick or too cold, methanol should be added (no more than 50g). The additions
to the paste should be done over a time of about 3 hours.
After completion, the beaker containing the reaction mixture should be allowed to stand in an ice water bath for another hour. The beaker is then
removed from the bath and allowed to warm slowly to normal temperature. The final volume of the mixture should be around 130 mL. After one hour at
room temperature, the mixture is placed on water bath, heated to 25 degC, and stirred for 30 minutes, then heated to 32-35C, and stirred for 30 more
minutes, finally being heated to 40 degC and stirred at this Temperature for another 15 minutes.
When the reduction reaction is complete, the solution is immediately filtered using a vacuum funnel, and filtering the solid product dry. The solid is
washed with 90 mL of water, then filtered. The solids left over are discarded. The filtrates (solution that dissolved the semicarbazide and passed
through the filter paper) are combined. The solution will contain around 10g of dissolved semicarbazide. The yield for the reaction of nitrourea to
semicarbazide at this point is 60%)
Dilute sulfuric acid (5% solution) is added to the solution until pH is correct. It is important that the resulting solution is not too acidic or
contain excess acid. It may be helpful to pre-cool the acid solution.
9.2g of Sodium Nitrite is separately dissolved in 125 mL water, and this solution is reacted with the semicarbazide sulfate solution (which contains
the equivalent of 10g semicarbazide). The addition should be slowly done, over the period of roughly ten minutes, the temperature should be kept below
20 degC, but ideally 10 degC is good. The reaction may give off some poisonous vapors just from it outside or in a fumehood. After this reaction,
immediately proceed to the final step, which is gradually added to the pre-chilled concentrated solution of sodium hydroxide.
The resulting solution contains sodium azide, along with byproducts of sodium carbamate, and sodium sulfate (much of it will precipitate out the solid
crystals on the bottom below 15 degC).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
ROFLOL
You are affraid to work with H2N-NH2 what is not a real problem and you prefer to work with
"The reaction may give off some poisonous vapors just from it outside or in a fumehood."
--> The poisonous vapors are HN3 what is about as toxic if not more than HCN (cyanhydric acid)!
I have worked with 80% hydrazine without particular précautions and yes it has a weird smell...so what?
NH3 is also toxic it was used as a war gas...if you can handle NH3 vapours from concentrated 35% NH3 solution...
--> Certainly you will have no troubles with N2H4 (even 80% - mono hydrate) what is less volatile than saturated NH3 solution...because N2H4 is a
liquid at STP while NH3 is a gas...so vapor pressure is much lower.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Hahaha that reply made me laugh Philou.
Just perform the hydrazine synthesis step outside if you don't have decent fumehood and cover the beaker with plastic wrap if you must like Nile red's
youtube video on it. I have done this reaction a few times with no issues.
Once you have converted the hydrazine to the sulfate salt it is only really a danger if you ingest it until it is dissolved in alcohol.
Be good, otherwise be good at it 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I'm glad I made your day   
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
|