| Pages:
1
2 |
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
Easy pyridine synth. found in a paper, does it work?
I am in need of larger amounts of pyridine and therefore the niacin decarboxylization, well known in this forum, is no option. At the bottom of this
thread
http://www.sciencemadness.org/talk/viewthread.php?tid=62385#...
someone posted several references for pyridine synthesis. Especially this one
http://gallica.bnf.fr/ark:/12148/bpt6k90810b/f29.image.langE...
woke my interest. Although it is from 1891 it seems to be ok.
The author claims that a mixture of pyridine derivates can be obtained from heating glycerol with ammoniumphosphate and continously destilling of
products.
According to the author for 500g of glycerol around 6-8 hours are needed to complete the reaction.
He fractional destilled 2.4kg of the product and obtained around 800g fraction below 120°C which seems to contain mostly pyridine and another 800g
fraction at 140-145°C which seems to be of 3-picoline.
This sound amazing since this offers a pretty high yield from very cheap educts. I have glycerol at hand but unfortunately no ammoniumphosphate. Has
anyone yet tried this?
Check this paper as well:
http://journals.tubitak.gov.tr/chem/issues/kim-14-38-4/kim-3...
They give a lot of detailed information and point out that many other ammonia salts can be used (diammonium phosphate is said to work best).
Additionally this seems to be a one pot synthesis.
[Edited on 25-6-2017 by subskune]
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 131
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
The mentioned latest paper saying: The procedure is not only ionic, but free radical mechanism too . You will obtaining a lot of pyridine isomers
and stinky tarry mass. The separation and purification of these will be a big challenge for you. (big suck) But never mind. Try it out, thats the
chemistry.
[Edited on 25-6-2017 by Aqua-regia]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The Chichibabin pyridine synthesis results from a Michael addition of acrolein to acetaldehyde enamine. Maybe you can accomplish this by heating
glycerol with acetaldehyde ammonia trimer?
But the "correct" way is probably to make acetoacetate by standard methods and do this:
https://en.m.wikipedia.org/wiki/Hantzsch_pyridine_synthesis
[Edited on 25-6-2017 by clearly_not_atara]
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
I found this in almighty wikipedia:
| Quote: |
3-Methylpyridine is produced industrially by the reaction of acrolein with ammonia:
2 CH2CHCHO + NH3 → 3-CH3C5H4N + 2 H2O
This reaction also affords substantial amounts of pyridine.
|
I think this is the major reaction that is takung place, taking the ammonia from the salt which is acid catalyst for the acrolein at the same time.
(Correct if I am wrong)
@atara:
since I plan to make around a liter of pyridine the reaction needs to be as cheap and simple as possible. This makes the hantzsch synthesis not
economical mostly because of the acetoacetate.
@aqua-regia:
Yep, the first one says that around 2/3 of the product is derivates and waste. However 1/3 are 3-picoline which could be converted to pyridine
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 131
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
It is hard to believe that you can with these methods pure pyridine producing. I am still wondering for your result.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2755
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
It depends on what the pyridine is being used for. If it is just being used as a base and solvent, having some 3-methylpyridine mixed with pyridine
might not be a problem. If you are doing chemistry on the pyridine, then starting with a mixture will be lead to a mess. For a large amount, it
might be more practical to just buy some, it can't be that hard to find a source for a liter somewhere.
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=20145&...
https://www.youtube.com/watch?v=r-xt24lBbrs
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I just ran a Google search for "niacin bulk" and found a few people selling it by the kilogram. How much pyridine do you actually need, anyway? More
than kilograms?
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
I am back home now and soon I'll start on the pyridine.
The pyridine will be used as a solvent. I don't think I'll need more than a liter. The niacin decarboxylation is quite simple but even "bulk"
suppliers do sell 2.5kg for about 60$ which is 10 times more expensive than glycerol and np fertilizer and I have to make the copper carbonate myself
which takes some time.
[Edited on 6-7-2017 by subskune]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by subskune  | I am back home now and soon I'll start on the pyridine.
The pyridine will be used as a solvent. I don't think I'll need more than a liter. The niacin decarboxylation is quite simple but even "bulk"
suppliers do sell 2.5kg for about 60$ which is 10 times more expensive than glycerol and np fertilizer and I have to make the copper carbonate myself
which takes some time.
[Edited on 6-7-2017 by subskune] |
The copper carbonate is probably the easiest and quickest step here, and will precipitate from solution and can be collected on the filter.
Na2CO3(aq) + CuSO4(aq) ==> CuCO3(s) + Na2SO4(aq)
The copper sulfate can be had cheaply from hardware stores as root killer.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Another approach could be the 1,5-dienamine formation from condensing glutaraldehyde and hydroxylamine with removal of water.
I don't have a reference on hand, but I am sure one could be found.
EDIT: I found a reference to this procedure, but it mentions the cyclization of glutaraldehyde with hydroxylamine is rare, with only one example
appearing in the literature involving another 1,5-dicarbonyl compound.
https://books.google.com/books?id=76MDkv1-KEUC&lpg=PA278...
[Edited on 6-7-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Nicotinamide to Pyridine via Hofmann Rearrangment/Reduction
This just popped into my head and I am not sure of the feasibility.
Would pyridine be produced by performing a Hofmann rearrangment on nicotinamide to 3-aminopyridine, followed by diazotization with nitrous acid to
3-diazopyridine (di)chloride. This could then be reacted with ethanol to produce what believe I would to be pyridine, acetaldehyde, N2, and
HCl.
The diazo compound should also be able to be reduced to pyridine using something like NaBH4, or similar.
I have read that benzenediazonium chloride will react with ethanol to produce benzene, where the ethanol reduces the diazonium compound and is
oxidized to acetaldehyde. I also read that methanol does not work analogously in this reaction. I will see if I can dig up where I got this.
EDIT: Added reference for Hofmann rearrangment of Niacinamide.
Niacinamide to 3-aminopyridine
http://orgsyn.org/demo.aspx?prep=cv4p0045
I also see Niacinamide for sale online at $109.96 for 5kg.
[Edited on 6-7-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
In theory making copper carbonate is really simple but every time I do these double replacement reactions I have to deal with blocked filters
impurities and partially wet product especially with a not heat resistant product. In my opinion it is one of the last things I want to do on kg
batches. On the other hand I am already looking out for root killer since this is some backup plan if I don't manage to buy or synthesize pyridine on
other ways.
I really dont't think that Hofmann rearrangement, diazotization and reduction leads to a cheap and simple process but for small quantities this might
be worth a try.
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
you might have more luck than with that crazy glycerol method by heating hot enough so that it decarboxylates, in pyrrole or a solution containing
that heterocycle, a dihalo acetate salt.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I found an OTC source of pyridine available to members in the USA.
This product contains 10% pyridine by weight.
http://www.domyownpestcontrol.com/nyguard-igr-p-423.html
It's an expensive route to pyridine. There might be other products that are more concentrated.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Doesn't that product have a long chain of ethers attached to the pyridine?
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Should have looked at the SDS first...
You are absolutely right, JJay. I should have looked at the SDS first, as it states the active ingredient is Pyriproxyfen, a pyridine-based pesticide.
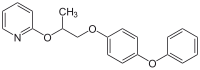
You could look at it this way, it's also a source of phenol, hydroquinone, propylene glycol, and 2-pyridone. It's funny as you wouldn't even be able
to get pyridine from it since it would take on its more stable form, since this is actually a masked amide.
[Edited on 10-7-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
A friend tells me you can make the Hanzsch ester from acetoacetate esters and hexamine. So that just got easier, I think.
Maybe acetoacetate could be made by alkylating the salt of 3-hydroxybutanoate, followed by oxidation? IIRC this acid is a common natural product,
though I'm struggling to think of a source. That's easier than a Claisen.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
@atara: Got a reference for hexamine working for dihydropyridine synthesis? I have acetoacetates, and while I also have formaldehyde hexamine is so
much nicer to work with. I'll add it to my ever-growing list of experiments to try one day.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
https://www.jstage.jst.go.jp/article/bpb/26/8/26_8_1211/_art...
Hiprex is hexamine hippurate
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Following up on the idea from @Loptr, there are easier/more accessible Hofmann rearrangements than using Br2 etc. I've had success with ordinary
chlorine bleach and NaOH. See:
http://www.sciencemadness.org/talk/viewthread.php?tid=65969
As for the following diazotization and reduction to hydrogen, there is US patent 4577046 claiming that hydrogen peroxide will suffice. I tried to
attach the patent, but not having any success.
I might attempt this series of reactions in the next week or so.
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
@atara:
I think the Claisen condensation is a pretty simple way to acetoacetate since I have propylene carobonate
Hexamin is basically the esbit fire starter. That stuff is pure and super cheap.
How to make pyridine out of them now? Is there any procedure or instruction or is this real chemistry?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The paper I linked has a free fulltext. The link is at the bottom of the page but it's kinda small:
"A constant volume (2.0 ml) of 5% solution of EAA or MAA in n-propanol was mixed with ser- ial concentrations of hexamine or hiprex in phosphate
buffer (2 ml; 0.1 M; pH 6.0) and heated on an oil bath at 100 °C for 60 min. The greenish yellow solution obtained was made up to 10.0 ml using
phosphate buffer. The absorbance was mea- sured in the spectrum mode and photometric mode (370 nm). Fluorescence spectra were collected using a slit
width of 4 nm and an excitation wavelength of 370 nm. The emission maximum was observed at 465 nm.
There's no workup here but the formation conditions are nicely laid out. The final product is apparently fluorescent which is both cool and gives you
a way to ensure it's working.
[Edited on 11-7-2017 by clearly_not_atara]
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
Thanks I somhow did oversee the link. The thing is how to get pyridine out of that now? Lets assume I have a solid amount of this esters. I need to
break the esters then decarboxylate and then? Can this stuff be dealkylated or do I need to oxidize it first and then decarboxylate all of this. I
mean that will never work: Removal of 4 acid groups.
Btw I ran across this article during my pyridine research:
https://en.wikipedia.org/wiki/M5_fiber
This looks like tetraaminopyridine which can be made from that esters over the intermediate tetracarboxypyridine and hoffmann rearrangement. Well that
semms to be dreaming
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | I ran across this article during my pyridine research:
https://en.wikipedia.org/wiki/M5_fiber
This looks like tetraaminopyridine which can be made from that esters over the intermediate tetracarboxypyridine and hoffmann rearrangement. Well that
semms to be dreaming |
I had actually posted a thread about M5 synthesis once, consensus opinion was basically "it's tough" but now that you mention it maybe oxaloacetic
acid bis-amide would give the 1,4-dihydropyridine-2,3,4,5-tetracarboxamide which is a more direct precursor.
| Quote: | | Can this stuff be dealkylated or do I need to oxidize it first and then decarboxylate all of this. I mean that will never work: Removal of 4 acid
groups. |
Removing the two carboxylate groups is enough; the product 2,6-collidine is useful in nearly every application pyridine is, and is usually a superior
ligand. I wouldn't call it a disadvantage unless you're depending on a low boiling point or something.
[Edited on 12-7-2017 by clearly_not_atara]
|
|
|
| Pages:
1
2 |