| Pages:
1
2 |
3DTOPO
Hazard to Self
 
Posts: 64
Registered: 14-2-2016
Member Is Offline
Mood: No Mood
|
|
That would only work with an inert atmosphere or vacuum. Somewhere over 600C carbon sublimes, and the hotter it is, the faster it goes.
|
|
|
3DTOPO
Hazard to Self
 
Posts: 64
Registered: 14-2-2016
Member Is Offline
Mood: No Mood
|
|
BTW: I use this castable refractory for a steel melting furnace - its really nice stuff. Its rated to 1760C. I use that for the hot face. Not quite 1800C - but darn close
- I imagine it would last quite a while.
Backed with insulating refractory for insulation. Its rated for 1648C.
The only higher rated stuff (for working in earth's atmosphere) that I aware of is MgO and ZrO refractory, but its expensive and difficult to source.
Perhaps a thin layer of ZrO for your hot face, then the 1760C followed by the 1648C insulating refractory.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by 3DTOPO  |
That would only work with an inert atmosphere or vacuum. Somewhere over 600C carbon sublimes, and the hotter it is, the faster it goes.
|
Yes, I discuss the necessity and use of an argon inert atmosphere several times on this thread - not a real problem IMHO, a really high temperature
furnace is going to be enclosed anyway due to the intolerable loss of heat otherwise and argon is cheaply and readily available to provide inert
atmospheres for welding.
An inert atmosphere is likely required for most processes of interest at extremely high temperature - most refractory metals for example oxidize
readily far below their melting points.
An additional benefit: argon has a lower thermal conductivity that air (or nitrogen) and thus improves the insulation factor.
Reference indicating that carbon sublimes at atmospheric pressure at anything like 600 C? This is the first I've heard of it. Its widespread use in
high temperature crucibles and the like seems to refute this. Most sources claim it has one of the highest sublimation temperatures.
About that which we cannot speak, we must remain silent.
-Wittgenstein
Some things can never be spoken
Some things cannot be pronounced
That word does not exist in any language
It will never be uttered by a human mouth
- The Talking Heads
|
|
|
3DTOPO
Hazard to Self
 
Posts: 64
Registered: 14-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by careysub  | Quote: Originally posted by 3DTOPO  |
That would only work with an inert atmosphere or vacuum. Somewhere over 600C carbon sublimes, and the hotter it is, the faster it goes.
|
Reference indicating that carbon sublimes at atmospheric pressure at anything like 600 C? This is the first I've heard of it. Its widespread use in
high temperature crucibles and the like seems to refute this. Most sources claim it has one of the highest sublimation temperatures.
|
I may have used the wrong term, but graphite crucibles are definitely consumable, and the higher temperature I use them at, the shorter they
last. I will be lucky to use a brand new thick-walled high purity graphite crucible 10 times when melting copper before it is used up. Clay graphite
crucibles last longer than the high purity crucibles because of their clay content (usually alumina based clays).
I guess it is oxidization that is the issue and not sublimation?
http://jes.ecsdl.org/content/110/6/476
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
How tolerent are moly elements to water vapour, lots of water vapour. I was led to believe they are fine in an oxidizing atmosphere.
How delicate are moly d when it comes to dust etc from use in a more diry furnace? I thought dust etc would not bother them.
I have purchased two moly d elements cheap on ebay, about 160 dollars). The heating part is around 200mm long and 6 mm dia. They run at around 160
amps (200 amps is there max current.) They are rated at 1800C.
I am hopeing to run them off a large variac and a buzz box welder transformer. I will perhaps heat the furnace to 600C or so simply by placing some
electric heater elements (domestic, 'pencil' elements) into the furnace which can then be removed as the buzz box is brought into action. The
difficult part with moly d is when they are cold. Very low resistence.
There is details of running mold d's at the following link
http://www.wetcanvas.com/forums/showthread.php?t=490969
Would the following elements , quartz halogen heater elements, work as heaters in a furnace.
http://www.ebay.co.uk/itm/3-Pack-400W-Elements-Halogen-Heate...
http://www.ebay.co.uk/itm/RTC-LA-306-LA-304-IR-FURNACE-INFRA...
The second link are actually furnace elements. More expensive than the domestic heater spares.
The spares are cheap and would be great for agressive atmosphers (I presume). I have purchased 6 and we will see.
Perhaps I will need to attach Nickel wire to each end?
Yob
|
|
|
3DTOPO
Hazard to Self
 
Posts: 64
Registered: 14-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by yobbo II  |
How tolerent are moly elements to water vapour, lots of water vapour. I was led to believe they are fine in an oxidizing atmosphere.
|
Apparently not good according to this paper.
Quote: Originally posted by yobbo II  |
How delicate are moly d when it comes to dust etc from use in a more diry furnace? I thought dust etc would not bother them.
|
I don't know exactly, but when I was researching them, I read that they are susceptible to dust and best used in a clean room. I guess the cleaner
they operate, the longer they will last.
It states on that page they are rated up to 1000C.
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Balls. There goes the Moly D idea out the window!
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Researching refractory metals I came across this report about greatly reducing the oxidation of refractory metals by phosphiding them.
Phospiding is done by heating the metal in an oven with a phosphorus atmosphere.
Not too attractive a process.
Yet, thinking about, methods of producing phosphorus using aluminum and any of many phosphates at not too high a temperature have been detail on this
site. The main problem is containing the phosphorus while also getting it out of the reaction vessel safely.
Perhaps a sealed vessel phosphiding process could be devised, in which the phosporous is produced in the sealed vessel via the aluminothermic
reaction, and then destroyed through oxidation to P2O5 before it is opened.
From the report:
ABSTRACT
Samples of niobium, tantalum, molybdenum and tungsten metals
have been given phosphided cases by heating in a phosphorous
atmosphere. The samples were subsequently exposed to air at an
elevated temperature. In tests at 800°C, the phosphided samples
resisted air oxidation markedly compared to the pure untreated
samples. The phosphided cases were only a few microns thick yet
oxidation resistance of all these samples extended for several hours.
Phosphided samples with 90° edges did not resist oxidation at the
edges as well as rounded edge samples.
And
Phosphide cases have been prepared on a number of metals by the
direct reaction of elemental phosphorus vapor on the massive metal.
Investigators at the Nuclear Corporation of America (4) have prepared
phosphide cases on sheets of four refractory metals, yttrium, zirconium,
hafnium and molybdenum by this means at temperatures ranging between
600° and 1000°C. Although the phosphiding treatment was for periods
of up to 20 hours, no values of total phosphorus pick up or phosphide
case thickness were reported.
Attachment: Oxidation studies of some refractory metals.pdf (645kB)
This file has been downloaded 821 times
About that which we cannot speak, we must remain silent.
-Wittgenstein
Some things can never be spoken
Some things cannot be pronounced
That word does not exist in any language
It will never be uttered by a human mouth
- The Talking Heads
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
The Moly D is now back in the window........
My understanding of Moly D was that it formed a coating of SiO2 on the outside which protected it. This coating has to form the first time the
elements are fired up. If the elements are held at a 'low' temperature the first time they are fired up (with or with or without extra water vapour)
in air they will oxidize.
Once they are properly fired up and 'cured' on their first run they should be OK from then on (minus dust).
Fused quartz is OK in a water vapour oven and thats what is on the outside of the MD elements.
@Careysub
I have to get my oven going before I can make P!
|
|
|
3DTOPO
Hazard to Self
 
Posts: 64
Registered: 14-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by yobbo II  |
The Moly D is now back in the window........
My understanding of Moly D was that it formed a coating of SiO2 on the outside which protected it. |
True, but the synopsis of the paper I referenced states:
| Quote: |
It is shown that the oxidation rate increases drastically in the presence of water vapour, and the growth Of MoO3 crystals on the oxide surface
increases considerably. The different re.-ions in the oxide cross-section are Mo-depleted compared with the corresponding regions in the bulk when
oxidised in oxygen saturated with 10% water vapour. However., the samples oxidised in dry oxygen only shows Mo-depletion in some outer parts of the
oxide. Accelerated growth of the MoSi2-oxide layer during exposure in O-2+10%H2O compared to that in O-2 can be related to the fact that more volatile
Mo-species form in the presence of water vapour, resulting in a substantial loss of MoO3 from the inner part of the oxide. The voids left behind are
not healed by the silica at this low temperature, which leaves the oxide with an open structure. As a result, the oxidation rate increases.
|
That doesn't sound good.
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Radient Energy Furnace
Has anyone tried this?
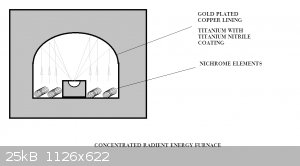
I'd think you could get some serious W.m-2 at the focal point. It's a matter of just how much you want to melt dictates how big the overall
furnace is. But a smallish (microwave oven) size could have a target of 20-30g???
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Unfortunately even if the reflector is 100% reflective, perfectly focused on to all sides of the target and the target is suspended in a vacuum such
a scheme can only heat the target to the same temperature as that of the elements.
|
|
|
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
MAPP gas burns a 2000C in air
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Super Kanthal heater 1800C for sale
It is very small. Would make a 'one brick hollowed out' furnace.
They don't come up very often on ebay at this price.
I am not the seller!
http://www.ebay.co.uk/itm/Kanthal-Super-1800-heating-element...
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Radiant energy furnace
A few days ago I had the opportunity to talk furnace design with a furnace engineer. I made a small deviation in our discussion to cover the
workability of the radiant energy furnace design. His software couldn't model the focusing effect of the reflective dome, but an approximation to
assume high levels of gain like any solar concentrator has, meant the target saw some extraordinary temperatures. His concern became one of there not
being any material that would cope with the temperatures. We modeled graphite as a crucible surrounded by zirconia surrounded by mica in fire brick.
The floor of a 0.5m^2 oven with kanthal wire at an optimum spacing for steady state operation at 1500C surrounded by kiln designed to be losing
300W.m^-2 gave a power rating of around 3.5kW for the elements. The elements gave off most of their energy as radiation once the furnace was at 1500C.
Some of the details were lost to me, I wasn't looking at his screen and didn't get to see how the software interpreted the models,or even what was
supplied as inputs, so I'll call this a sort of back of an envelope calculation until someone gets to either do empirical work or better modeling.
But it boiled down to the elements giving off about 2kW.m^-2 in radiation from the array on the floor and focused from the reflector, with a
collection area of 0.5m^2, to a target of 2x10^-3 m^2. (25mm radius) gives a magnification factor of about 250.
If the incoming radiation is at 1500 W.m^-2( because the whole floor area is not radiating at the energy level of 2kW.m^2) multiplied by 250 then the
target should be receiving 125kW.
Anything that is getting that sort of energy directed at it is going to be seriously hot.
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
What would you make the reflector from?
You would need to start with a lower temperature (1000C?) in order to have a refector that could cope. A metal (shiny) reflector would need to be in
an inert atmosphere to stop it oxidizing and becoming non reflecting.
All above IMO, I really don't have clue.
I ordered a zirconium oxide rod from ebay (about 20 dollars) and hope to make a 'one brick' oven that will go to approx 1800C.
No idea what to do when it is made. Measuring the temperature and controlling the temperature would be a problem.
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
My original idea was Ti coated with TiN, an excellent reflector of IR. Also chemically inert to temperature induced oxidation. CVD Si is an option.
This is getting way past backyard science I have to admit.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Well, trying to return to more of a backyard approach....
Crucibles are always a problem, and about the best you're going to find commercially made are yttria. It's easy to find small-volume ones, larger ones
seem to be more difficult to find.
Dollar for dollar, you simply aren't going to find a more cost-effective solution than an optical pyrometer for temperature measurement. This is also
good if you need to cheap-out and not provide an inert atmosphere. In fact, using one with an inert atmosphere is the trick.
Not providing an inert atmosphere makes no sense. As was mentioned a number of posts back, it's not hard to achieve and it isn't expensive. I would
think that unless the furnace is just a passing toy, this would be the only way to go. And really, what do you even want to heat in air?
Inert gas opens up a way to get away from the many, many restrictions you get with molybdenum disulfide, silicon carbide, and other specialty
conductive ceramics.
1) the control of flow of electricity through the carbon resistance heaters is straightforward. Simple even.
2) the elements are not subject to cracking, they require no special conditioning, they tolerate dust. They are much cheaper than the ceramic options.
I'm a firm believer in taking cues from established procedures when you're trying to replicate industrial or laboratory grade results. Carbon heaters
have been used for a long time to achieve very high temperatures.
The drawback is that the elements are not as clean as the ceramics.
There are a myriad of choices. The only way you can really choose one rationally is if you look at the intended use first.
[Edited on 5/25/2017 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I think that the comment by wg48 should be carefully considered,
Quote: Originally posted by wg48  | | Unfortunately even if the reflector is 100% reflective, perfectly focused on to all sides of the target and the target is suspended in a vacuum such
a scheme can only heat the target to the same temperature as that of the elements. |
... the target will heat up to the same temperature as the source as it will then be in equilibrium,
(e.g. real solar furnaces only reach about 4,000 K from a 6,000 K source)
This is a fundamental constraint. (I believe)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by yobbo II  |
I ordered a zirconium oxide rod from ebay (about 20 dollars) and hope to make a 'one brick' oven that will go to approx 1800C.
No idea what to do when it is made... |
Wiki says "white heat" is ≥1315°C. With that heat you can make phosphorus from dried H3PO4/C. I'm not sure what you would use for the retort,
possibly steel or a ceramic. (ref: The Chemical Process Industries by Shreeve)
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Before I give up on this one, consider the old light bulb, the filament gets to nearly 3kK (3000K) but the bulb is almost touchable. I don't think
that the oven could ever reach anything close to the same temperatures as the target.
And by extension from the bulb logic, if you were to try to heat a furnace of 0.5 m^3 with a 5cm^3 source at 3kK, you'd be lucky to get the walls to
1kK. The oven would be leaking energy at somewhere around 300-700 W.m^-2 at this temperature so they could never attain any where near the source
temperature.
Ponder again the prospect of pouring 125kW into something reasonably small....hmmmm
|
|
|
floridajohnny
Harmless

Posts: 2
Registered: 31-5-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mikeehlert  |
Oil Tankers use their own exhaust gas (CO2, CO, N, C, & hydrocarbons) to inert the cargo tanks. Works well enough to prevent most explosions.
Mike |
Is this true 100% of the time???
|
|
|
Texium
|
Thread Moved
27-11-2023 at 11:26 |
| Pages:
1
2 |