| Pages:
1
..
54
55
56
57
58
..
77 |
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Wow nice, are they transparent to some extend ? On the photo it looks like you might be able to see through them a bit ?
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Thanks. Ill check and let you know, as i can't remember how they were in person.
|
|
|
CrystalCage
Harmless

Posts: 7
Registered: 22-11-2015
Member Is Offline
Mood: No Mood
|
|
Made some CuCa(CH3COO)4.6H2O
Really hard to make, 4:1 Ca:Cu ratio does not always work, several failed attempts has been made.
The solution is quite supersaturated.. Need to dilute again the mother liquor to obtain the seed crystals.

|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
HeYBrO and Crystalcage, cool copper crystals! Copper Calcium Acetate is definitely on my todo list. And HeYBrO's would be too if I had Bipy....:/
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
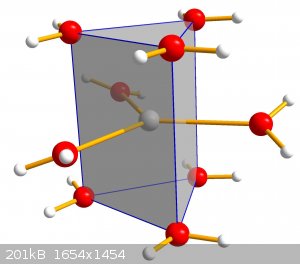
Well this is not really a photo but I still love the structure. Had to explain why hydrates PdO can be dehydrated for YouTube and compared it to the
dehydration of CaCl2x6H2O when I found out that for some reason there is no good picture of the Hexahydrate anywhere on the web
which I found really sad. So I took the crystal structure data from the literature and simulated the tricapped trigonal prismatic structure again.
You can see how each Ca has a coordination number of 9. Along the axis many of these stack on their triangular surfaces so you end up with
6/2 + 3 caps per Ca which equals 6 H2O and thus is called hexahydrate.
As you can see there is no Chloride to be seen. I didn't add them to make it easier to see but they are surrounding this structure with hydrogen bonds
towards the water hydrogens. If you want to dehydrate that thermally there is no real contact between Ca and Cl which is why other compounds form as
well.
I find this structure really nice because you don't expact something like that for a simple Calcium 
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
CrO3, the largest crystal is ~10mm across.
 
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Are you sure that is CrO3? I thought the crystals looked like needles....
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Tried my hand at synthesizing xanthopurpurin a little while ago and my first sample of purified material crystallized in a strange, but very pretty
way.

|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Wow that's beautiful! That would make a great desktop background.
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
I had a concentrated sodium sulfate solution sitting in a beaker on the bench for a few days. When I checked the lab today I noticed some very
beautiful crystals, this was the best one.
   
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice crystal! To be honest that's one thing ive never really tried to crystallize, I'll have to try it. Was attempting to grow epsom salt crystals but
accidentally left it on the hot plate the other day...now I have some nice anhydrous epsom salt...
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
Panache, how did you crystallize that CrO3?
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Beautiful color 
[Os(Thiourea)6]3-

|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Some potassium chloride crystals slowly grown from water.
 
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Molten silver on a piece of drywall; you can see the reflection of the room

My element collection in no particular order

Bubble of boric acid as I dehydrate it to make boron trioxide

Crystals of boric acid on a beaker

[Edited on 26-5-2017 by Plunkett]
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
That element collection shot is pretty cool. I like it a lot. 
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
A few flowers I was keeping, with stems split between two water sources, each dyed a different color.
 
I took a few pictures under the microscope. The first shows the sharp partitioning in the flower petals. The second is interesting because it shows an
upwelling of one color in a sea of the other!
 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Some zinc sulfate I finally arsed myself to recrystallize it from its solution, after sitting in a closet for 2 years.

|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
One picture is bunch of copper(II) acetate crystals.
In next pictures is my attempt to double phantom crystal, chrome alum crystal in chrome alum+ KAl alum mixture in KAl alum. The inner black crystal is
not visible tho so it's more like normal phantom crystal.
  
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Here's some crystallized copper acetate from a spill:

(Open in a new tab for the full experience  ) )
And this is thermochromism from flower pigments. The alcoholic extract of lilac petals changes color when heated (and cooled). The first photo is at
room temperature, In the second the right test tube has been heated to ~70C, and the third photo is when it has cooled back down to room temp.
 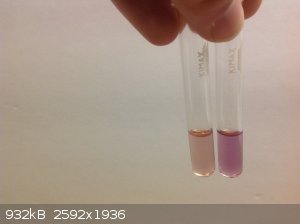 
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
I'm pretty sure most of the people here have seen the various oxidation states of manganese, but I'll post it anyway. I particularly like the +2
oxidation state because of the slight gradient from dark to light pink then to clear. The +7 and +6 are way too dark (probably too concentrated of
KMnO4 solution), and the +4 simply precipitated  . .

|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
First is a sealed vial containing reacting bromine and sulfur in n-butanol. The end products will presumably be sulfuric acid and bromobutane.
Second is a pool of galinstan with other metals dissolved in it from prior experiments. They form an oxide crust on its surface.
Third is an attempt to oxidize phenylalanine past the amine stage. It ended up forming a sphere at the center of the test tube.
  
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
My first success in making K2[OsCl6]. As mentioned before I am not using pure OsO4 but a very contaminated solution
which also has other reactive compounds from the dissolution process of Os metal in it. So I cannot simply copy experiments but have to redesign each
of them. No matter what text I used I was not able to make it directly but had to go to [OsO2(OH)4]2- first which is
insoluble in Ethanol and can be cleaned that way and then turn this into the chloro complex. Still, I am pretty satisfied with the results, an
insoluble red powder. The problem is I probably added too much or heated it too long as it decomposed a few seconds afterward.
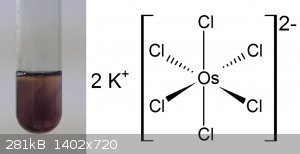
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
My attempt at the Copper/Acetone "lamp" experiment (ketene generator), as well as my first attempt at synthesizing picric acid from acetylsalicylic
acid(recrystalization), and my twenty-fifth (unsuccessful) attempt at nitrocellulose:
https://i.imgur.com/x3Nkv04.jpg
https://i.imgur.com/dGigrRw.jpg
https://i.imgur.com/QLUNuzD.jpg
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Potassium chlorate crystals from my second batch(9.22g):
https://i.imgur.com/Cah6s84.jpg
Hydrazine sulfate precipitating (24.24g from using hand stirring):
https://i.imgur.com/2sktdCU.jpg
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
| Pages:
1
..
54
55
56
57
58
..
77 |