edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
TriazidoNitromethane CN9NO2
I was watching videos on You tube, was that I came across the chloropicrin (CCl3NO2) it is trichloronitromethane, a compound made by reaction between
nitromethane and sodium hypochlorite solution .
NaOCl + CH3NO2 --> CCl3NO2 + NaOH
Chloropicrin is little soluble in water 2g/1000g , is extremely toxic and volatile, seeing its molecular structure, Three chlorine atoms connected to
a carbon and nitro NO2 .... Equal as 1,3,5-Triazido-2,4,6-trinitrobenzene C6(N 3)3(NO2)3 which is obtained by a reaction in solution of
trichlorotrinitrobenzene with sodium azide .
C6Cl3N3O6 + NaN3 --> C6(N3)3(NO2)3 + NaCl
in this image show how the chlor is substitued by azide N3
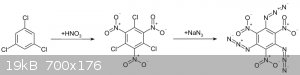
And if use instead of trichlorotrinitrobenzene, use the trichloronitromethane (chloropicrin) in a solution and sodium azide, the triazidonitromethane
CN9 (NO2) will form ????
CCl3NO2 + NaN3 ---> CN9(NO2) + NaCl
Imagine how powerful this compound can be !! High nitrogen content 76% !! And still the perfect OB 0% everything after detonation is N2 and CO2 ...
Anyone with more knowledge of chemistry would know if this reaction is possible ?? I believe no one has ever tried due to the extreme toxicity of
trichloronitromethane (chloropicrin) . What you say about it ??
Other exemplo Chlor substitued by N3 Azide
cyanuric triazide C3N12

[Edited on 9-3-2017 by edsonmartins113]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Triazidomethane and tetrazidomethane are hell unstable...
My guess is that triazidonitromethane would also be...but worth a try in tiny mini quantities and for sure into a soft plastic container (avoid at all
cost hard plastic, glass or metals --> in case of detonation even if into solution --> schrapnels piercing flesh and bones...
There is also a possibility that the compound reacts intramolecularly or intermolecularly
O2N-C(N3)3 --> O=N-C(N3)2-N=O + N2 (reduction of NO2 into N=O and oxydation of N to N=O)
O2N-C(N3)3 + (N3)3C-NO2 --> O2N-C(N2)2C-NO2 (tetrazine) + N2
or formation of alcenic compounds like O2N-C(N3)=C(N3)-NO2 or (O=N-)2C=C(-N=O)2
last possibility NO2 nitro-nitrite rearrangement
O2N-C(N3)3 <--> O=N-O-C(N3)3
O=N-O-C(N3)3 --> NO(+) + (-)O-C(N3)3
(-)O-C(N3)3 --> O=C(N3)2 + N3(-)
O=C(N3)2 (very unstable) --> CO + N2
[Edited on 9-3-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | Triazidomethane and tetrazidomethane are hell unstable...
My guess is that triazidonitromethane would also be...but worth a try in tiny mini quantities and for sure into a soft plastic container (avoid at all
cost hard plastic, glass or metals --> in case of detonation even if into solution --> schrapnels piercing flesh and bones...
There is also a possibility that the compound reacts intramolecularly or intermolecularly
O2N-C(N3)3 --> O=N-C(N3)2-N=O + N2 (reduction of NO2 into N=O and oxydation of N to N=O)
O2N-C(N3)3 + (N3)3C-NO2 --> O2N-C(N2)2C-NO2 (tetrazine) + N2
or formation of alcenic compounds like O2N-C(N3)=C(N3)-NO2 or (O=N-)2C=C(-N=O)2
last possibility NO2 nitro-nitrite rearrangement
O2N-C(N3)3 <--> O=N-O-C(N3)3
O=N-O-C(N3)3 --> NO(+) + (-)O-C(N3)3
(-)O-C(N3)3 --> O=C(N3)2 + N3(-)
O=C(N3)2 (very unstable) --> CO + N2
[Edited on 9-3-2017 by PHILOU Zrealone]
Like this ?? Azide + nitro = furoxan
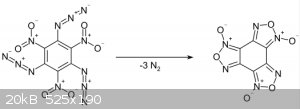
[Edited on 9-3-2017 by edsonmartins113] |
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yes that kind of reaction  on viccinal carbons via double link (sp2 carbon) or
maybe on geminal groups (thus onto the same carbon) on viccinal carbons via double link (sp2 carbon) or
maybe on geminal groups (thus onto the same carbon)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by edsonmartins113  | I was watching videos on You tube, was that I came across the chloropicrin (CCl3NO2) it is trichloronitromethane, a compound made by reaction between
nitromethane and sodium hypochlorite solution .
NaOCl + CH3NO2 --> CCl3NO2 + NaOH
Chloropicrin is little soluble in water 2g/1000g , is extremely toxic and volatile, seeing its molecular structure, Three chlorine atoms connected to
a carbon and nitro NO2 .... Equal as 1,3,5-Triazido-2,4,6-trinitrobenzene C6(N 3)3(NO2)3 which is obtained by a reaction in solution of
trichlorotrinitrobenzene with sodium azide .
C6Cl3N3O6 + NaN3 --> C6(N3)3(NO2)3 + NaCl
in this image show how the chlor is substitued by azide N3
And if use instead of trichlorotrinitrobenzene, use the trichloronitromethane (chloropicrin) in a solution and sodium azide, the triazidonitromethane
CN9 (NO2) will form ????
CCl3NO2 + NaN3 ---> CN9(NO2) + NaCl
Imagine how powerful this compound can be !! High nitrogen content 76% !! And still the perfect OB 0% everything after detonation is N2 and CO2 ...
Anyone with more knowledge of chemistry would know if this reaction is possible ?? I believe no one has ever tried due to the extreme toxicity of
trichloronitromethane (chloropicrin) . What you say about it ??
Other exemplo Chlor substitued by N3 Azide
cyanuric triazide C3N12
[Edited on 9-3-2017 by edsonmartins113] |
Hmmmmmm wonder where you saw that video haha
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Well in theory trichloronitromethane could undergo the azidation with sodium azide to form CNO2(N3)3, but i guess the compound would not have a great
density, also it might be so sensitive that its extremely hard to even isolate from a solution.
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
nitroform and cyanoform are damn difficult in the first place, req. low temo. afaik.
|
|
|
Chisholm
Hazard to Self
 
Posts: 62
Registered: 2-4-2017
Member Is Offline
Mood: No Mood
|
|
Order of addition and choice of solvent is important. If you add the chloropicrin to dry sodium azide without a solvent, the product will probably
decompose almost instantly and with great violence. Methyl azide alone is incredibly sensitive to shock and friction; one can only imagine three azido
groups and a nitro group.
Edit: Thank you, Philou. My bad.
[Edited on 4-5-2017 by Chisholm]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
A little clue is given by parent compounds like formyl-diazide (phosgene analogue...for sure very toxic) N3-CO-N3 or methylene azide (diazidomethane
CH2(N3)2).
Methyl azide (CH3-N3) and ethylene diazide (N3-CH2-CH2-N3) are not too sensitive but they must be treated with great respect because shock and heat
sensitive explosives...and also toxic since hydrolysable slowly by air moisture into alcohol and HN3 gas (more toxic than hydrogen cyanide).
@Chisholm "one can only imagine three azo groups and a nitro group"
It is not azo group (azo group is -N=N- like azomethane what is CH3-N=N-CH3)...==> AZIDO group 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Cyanoform hasn't been synthesized, has it??
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Cyanoform has been synthesized, it was originally thought just difficult to produce, but likely stable at RT. Turns out it isn't, it requires storage
at a little below dry ice range temperatures. -40 'C or less. The synthesis was done in 2015, and involves first cooling excess anhydrous hydrogen
fluoride to -196 'C, then adding calcium tricyanomethanide (cyanoform is highly acidic, and this anion is known, not sure if it requires cryogenic
storage or not, thereafter, the mixture is 'warmed' to -78 degrees 'C to allow dissolution of the tricyanomethanide, forming cyanoform and Ca(HF2)2
followed by subjecting the result to vacuum distillation over at least 12 hours in order to strip the excess HF, resulting in a difficult to purify
mixture of CH(C-=N)3 and Ca(HF2)2. This is stable, a colorless liquid, below -40 degrees 'C.
I wonder if other pseudohalogenoforms are stable, such as CH(N3)3 and rhodanidoform/thiocyanatoform, isothiocyanatoform, or the corresponding selenium
or tellurium homologs of ROC-=N. AFAIK nitroform is stable enough to be isolated using more conventional, (read-less damned obnoxious) means, but is
difficult to produce.
Are dinitrohaloforms (such as the dinitro versions of halopicrins) known?
Whilst it might not stick around too long, I'd guess that unlike some organic nitriles, cyanoform would be unholy toxic.
It had been detected previously in the highly diluted vapor phase, spectroscopically, but isolation of the compound itself took until 2015.
|
|
|