veganalchemist
Harmless

Posts: 30
Registered: 3-8-2010
Location: UK
Member Is Offline
Mood: No Mood
|
|
Electrolysis
Hi, I need to do some electrochemistry with magnesium electrodes.
I've got the electrodes but the current needs to be reversed every 15 seconds.
It's run at a constant current of 50 mA at about 2.7 V
Can't thinnk how to get the current to reverse without physically switching it by hand.
Could it not be switched every 60 seconds?
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
555 astable squarewave circuit? That should be pretty easy to put together.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Learn how to use arduinos (this will be a really useful skill in the future, inevitably. community support is great), and set up an H-bridge circuit.
You'll probably need at least two p-channel mosfets and two n-channel ones, as well as a way of measuring current. H-bridge circuits are usually
used to reverse the polarity of a DC motor, so that it can change directions, but it should also work for what you're doing too.
If that's too complicated, use a DPDT relay for the H-bridge circuit, which you can figure out how to wire up just by searching for those two terms.
I still recommend the arduino though, because of how configurable they are, and the fact that you can get the basic kind for around $6.
[Edited on 3/25/17 by Melgar]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Why?
There's likely to be an easier way.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
You can do the reverse switching with 2 MOSFETs and an oscillator which has two outputs A and A', where A = ~A'. Such an oscillator is easy to make
with a 555 circuit.
But my underlying question is, what do you need this for? What do you want to achieve?
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by woelen  | You can do the reverse switching with 2 MOSFETs and an oscillator which has two outputs A and A', where A = ~A'. Such an oscillator is easy to make
with a 555 circuit.
But my underlying question is, what do you need this for? What do you want to achieve? |
The power supply necessary would be non-standard, to say the least, but it could be done from mains AC using two half-wave rectifiers where the AC
voltage has been dropped down to like 5 volts with a transformer. You'd also need negative voltage regulators that could deal with the inevitable
short-circuits that tend to happen in electrochemistry.
Considering that 90% of the cost of buying electronic components is usually shipping and handling, it'd probably be almost exactly the same cost to
buy 5 power mosfets as it would be to buy one. The easiest way though, without resorting to a three-pin power supply, would probably be a DPDT relay
with the current controlled by a MOSFET, no?
I always try to answer these questions even if the OP doesn't actually need what he says he does, because it frustrates me to no end when I need a
solution to something, and I finally find a page where the OP asked EXACTLY the question I needed answered, only for the community to point out that
some other solution (that wouldn't work for me) would be better for him.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I think that a 2-pole changeover relay to switch polarity
e.g. 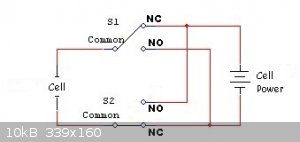
with the coil of the relay powered by something like this
http://www.ebay.co.uk/itm/Cycle-Adjustable-6-30V-Relay-Modul...
should work.
[Edited on 26-3-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
veganalchemist
Harmless

Posts: 30
Registered: 3-8-2010
Location: UK
Member Is Offline
Mood: No Mood
|
|
Got it sorted. Tried it out and it works.
Using a 15 s on and off reley to power a 2-pole relay.
Using a Cebek cyclic timer, 0.3 s to 60 s.
Not sure why you have to use magnesium but it says won't work with C, Pt, Cu, Ni, Cu or Pb.
Cathodic coupling of aliphatic esters.
Tetrahedron Letters, Vol . 36, No. 27. Pp 4805, 1995
|
|
|
Paulo99
Harmless

Posts: 2
Registered: 29-3-2017
Member Is Offline
Mood: No Mood
|
|
What do you want to achieve?
|
|
|