| Pages:
1
2 |
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
[Tests] Ammonium Chlorate NH4ClO3
I prepared 20g of ammonium chlorate by salt metathesis reaction between potassium chlorate and ammonium nitrate, dried in a pyrex form in a 45 ° C
shade heat, the product was very pure, solid white, I made several safety tests To check its stability, including the myth that it explodes in the sun
!! I did this a few times even with catalise and only resulted in slow decomposition ... OK !! What I really want to talk about is its explosive power
that caught my attention, I carefully prepared a mixture of dry ammonium chlorate with powdered aluminum (8g / 2g) 10g of the mixture, I wrappred on
paper with adhesive tape , The blend did not have 1/3 of its maximum density, I started the mixture with cap of 200mg HMTD in a 4mm sheet steel....
Result ???? A huge noise !!! Deafening !! The damage in the plate was equal to that of the ETN in the same density and confinement level, I estimate
it to have reached 5000 m / s ~ 6000 m / s ... Very powerful if considered by density and confinement, seeing the hole in the plate. .. Has anyone
done any testing with ammonium chlorate ?? Remembering that although it seems to be safe in some cases is good to be very aware !!
[Edited on 6-3-2017 by edsonmartins113]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
"Seems to be be safe" isn't something I would hold in my hands when it blows a dent in 4mm steel plate. I'm wondering what that would do with your
hands....
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I have made ammonium chlorate as well. Very little happened in sunlight, but it was very shock sensitive. The sample I tested wasn't even dry
and it still went off. It is with no doubt a very powerful explosive compared to other ammonium salts and aluminium will only make it go off easier.
If you value your fingers, don't do it again.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
It is also known to explode spontaneously upon storage.
I recall Shimizu describes in one of his books preparing some of the pure compound. All of the samples spontaneously ignited over the course of a few
days.
20g seems like a lot. I hope you are not storing any of it.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
For some applications, especially for instant testing on brisance is possible always preparation fresh 20g. Amateur praxis is basically only testing
samples. Hole in 4 mm steel ? From 10g only ? Very good result. Also I estimate 5000 m/s. However Without storage.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
underground
National Hazard
   
Posts: 703
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
How did you made Ammonium Chlorate lol. Ammonium Chlorate is much more soluble than potassium chlorate. Did you mean sodium chlorate ?
I even tried 1-2 times to make potassium chlorate with sodium chlorate and potassium nitrate without success, really have no idea how you did it lol
[Edited on 7-3-2017 by underground]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
How do you know?
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
I tried making this as well, but it failed ;-; But I made ammonium iodate a few times, but this stuff doesn't have explosive properties at all. On
heating it just completely dissapears into purple vapours, which still looks really cool: 2NH4IO3 -> N2 + I2 + 4H2O + O2
Suprisingly, there was still no wikipedia article about it, so I just made one: https://en.wikipedia.org/wiki/Ammonium_iodate it's mostly based on this 54 year old paper http://www.dtic.mil/dtic/tr/fulltext/u2/295766.pdf
Maybe I'll try to make ammonium bromate, that seems quite easy to do:
2KBrO3 + BaCl2 -> Ba(BrO3)2 + 2KCl
Ba(BrO3)2 + (NH4)2SO4 -> BaSO4 + 2NH4BrO3
But seeing as bromate is an even stronger oxidizer then chlorate, that's really scary to do.
|
|
|
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by underground  | How did you made Ammonium Chlorate lol. Ammonium Chlorate is much more soluble than potassium chlorate. Did you mean sodium chlorate ?
I even tried 1-2 times to make potassium chlorate with sodium chlorate and potassium nitrate without success, really have no idea how you did it lol
[Edited on 7-3-2017 by underground] |
1 mass molar of KClO3 and 1,0 de AN pure , Dissolve it until saturated , add ethanol at 96% ~100% , the KNO3 will precipitate , Take to the freezer at
-20°C , filter the KNO3 and dry the solution after filtering ..
[Edited on 7-3-2017 by edsonmartins113]
|
|
|
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
Because when I burned a sample on a piece of aluminum foil, there was no trace of residue, everything gas, orange fire without potassium ions that
leaves a flame of lilac , Melted 100 mg sample in foil aluminum and teste tube at 100 ° C, before decomposing it was possible to see that it was
totally colorless without any other salt like the KNO3 formed in the metathesis reaction
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I'm not convinced that your methatetical procedure would work at all nor that what you got was pure NH4ClO3...
1°) When checking solubility tables as function of temperature into water, one will see that amongst NH4NO3, NH4ClO3, KNO3 and KClO3...the order of
best solubility goes into the following order NH4NO3 > KNO3 > NH4ClO3 > KClO3... for all temperature range (0-100°C).
This means KClO3 being the least soluble...it will always precipitate first.
So you methatetical procedure simply doesn't hold the line and will not work at least into this world.
2°) When checking solubility tables as function of temperature into ethanol, one see that the same order of solubility applies...there are no data
for NH4ClO3 but there are for NH4Cl and NH4ClO4 what may give indications on the ones of NH4ClO3 by extrapolation from values of NaCl, NaClO3, NaClO4,
KCl, KClO3, KClO4, NH4Cl and NH4ClO4 (and NaNO3, KNO3, NH4NO3)...and here again KClO3 is the least soluble into ethanol (see table(*) I have made into
Excel below)...so your procedure here again shouldn't work.
Here in attachment a graphical view of this in pdf made from excel sheet and solubility datas from wikipedia and other sources...
Attachment: solubility chart KNO3-KClO3-NH4ClO3-NH4NO3.pdf (356kB)
This file has been downloaded 644 times
(*)
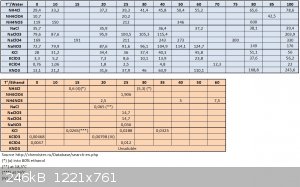
[Edited on 8-3-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
underground
National Hazard
   
Posts: 703
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I totally agree with PHILOU. Maybe you even havent got any NH4CLO3. If it would be that simple we would made NH4CLO4 from KCLO4. You have to use
NaClo4 instead of kclo4 to get a chance to work.
|
|
|
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | I'm not convinced that your methatetical procedure would work at all nor that what you got was pure NH4ClO3...
1°) When checking solubility tables as function of temperature into water, one will see that amongst NH4NO3, NH4ClO3, KNO3 and KClO3...the order of
best solubility goes into the following order NH4NO3 > KNO3 > NH4ClO3 > KClO3... for all temperature range (0-100°C).
This means KClO3 being the least soluble...it will always precipitate first.
So you methatetical procedure simply doesn't hold the line and will not work at least into this world.
2°) When checking solubility tables as function of temperature into ethanol, one see that the same order of solubility applies...there are no data
for NH4ClO3 but there are for NH4Cl and NH4ClO4 what may give indications on the ones of NH4ClO3 by extrapolation from values of NaCl, NaClO3, NaClO4,
KCl, KClO3, KClO4, NH4Cl and NH4ClO4 (and NaNO3, KNO3, NH4NO3)...and here again KClO3 is the least soluble into ethanol (see table(*) I have made into
Excel below)...so your procedure here again shouldn't work.
Here in attachment a graphical view of this in pdf made from excel sheet and solubility datas from wikipedia and other sources...
(*)
[Edited on 8-3-2017 by PHILOU Zrealone] |
Reacting by metathesis at a point where all of the salt converts, the KClO3 and ammonium nitrate dissolved in water ( satured solution ) to ammonium
chlorate and potassium nitrate , ... KClO3 + NH4NO3 -> KNO3 + NH4ClO3, using 1.0 mol of each salts .. The ammonium chlorate is quite soluble in
water, it is not soluble in pure ethanol , however NH4ClO3 is very well soluble in a mixture of ethanol with water , the KNO3 as KClO3 or any NH4NO3
is soluble in water pure , but not is on solution of ethanol with water ... I used ethanol to precipitate the KNO3 on solution ,after taked to the
freezer -15°C ~ -20°C the saturated solution of water with ethanol ,in that all KNO3 must precipitate leaving only the NH4ClO3 in the solution ...
it decomposes cleanly without leaving solid residue as of KClO3 / KCl in surfaces and more putting the drop of cold water in a sample of NH4ClO3
dissolves so easily and totally , is pure ou maybe almost pure , It does not matter that much ! 
[Edited on 8-3-2017 by edsonmartins113]
[Edited on 8-3-2017 by edsonmartins113]
[Edited on 8-3-2017 by edsonmartins113]
|
|
|
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by underground  | | I totally agree with PHILOU. Maybe you even havent got any NH4CLO3. If it would be that simple we would made NH4CLO4 from KCLO4. You have to use
NaClo4 instead of kclo4 to get a chance to work. |
was made this way
1.0 mol /1.0 mol KClO3 + NH4NO3 --> KNO3 + NH4ClO3 ... add water to make solution satured , wait reaction for 1 hr , add 900 ml ethanol , the
KNO3 will precipatate , filter the solution , take to freezer at -18°C for 3 hrs , filter again the the bit of KNO3 (note!) a bit of NH4ClO3 will
preciptate , but no problem ,Now the solution after filtering , is taken to dry at 40°C ,and this is NH4ClO3 ..... has low point melt around of
100°C e decomposes quickly without residue solid 
[Edited on 8-3-2017 by edsonmartins113]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The addition of the ethanol makes quite a difference. So, indeed it might be that you obtained NH4ClO3. What you did is more than a simple metathasis
reaction.
I believe that you had NH4ClO3, probably with a small fraction of potassium ions and nitrate ions in it as well.
I myself have done the experiment with NH4BrO3. I wrote a web page about that. See this thread: http://www.sciencemadness.org/talk/viewthread.php?tid=11958#...
[Edited on 8-3-17 by woelen]
|
|
|
underground
National Hazard
   
Posts: 703
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Anyone can tell my why my saturated solution of naclo3 and kno3 dont want to react?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
What do you mean? What reaction are you expecting? I don't expect anything
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
1.0 mol /1.0 mol KClO3 + NH4NO3 --> KNO3 + NH4ClO3 ... add water to make solution satured , wait reaction for 1 hr , add 90 ml ethanol , the
KNO3 will precipatate , filter the solution , take to freezer at -18°C for 3 hrs , filter again the the bit of KNO3 (note!) a bit of NH4ClO3 will
preciptate , but no problem ,Now the solution after filtering , is taken to dry at 40°C ,and this is NH4ClO3 ..... has low point melt around of
100°C e decomposes quickly without residue solid.
Is possible describe preparation in grams ? For example : In 100g H2O added 10g KClO3 + 10g NH4NO3. Wait 1 hour. (temperature during 1 hour ?)
Dissolved all (estimate) Why waiting 1 hour ? OK, next: Added 90g ethanol. In this time, KNO3 will precipitate out. Filtered, and use solution.
Solution content 90% NH4ClO3 + 10% KNO3. Go to solution on - 18 C. Again filtered and precipitate out is again KNO3. Solution content NH4ClO3 99% + 1%
KNO3 OK. heated on 40C , respectively evaporate H2O + ethanol. Dry crystals NH4ClO3 arises ? You can added values in grams? Thanks.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  |
1.0 mol /1.0 mol KClO3 + NH4NO3 --> KNO3 + NH4ClO3 ... add water to make solution satured , wait reaction for 1 hr , add 90 ml ethanol , the
KNO3 will precipatate , filter the solution , take to freezer at -18°C for 3 hrs , filter again the the bit of KNO3 (note!) a bit of NH4ClO3 will
preciptate , but no problem ,Now the solution after filtering , is taken to dry at 40°C ,and this is NH4ClO3 ..... has low point melt around of
100°C e decomposes quickly without residue solid.
Is possible describe preparation in grams ? For example : In 100g H2O added 10g KClO3 + 10g NH4NO3. Wait 1 hour. (temperature during 1 hour ?)
Dissolved all (estimate) Why waiting 1 hour ? OK, next: Added 90g ethanol. In this time, KNO3 will precipitate out. Filtered, and use solution.
Solution content 90% NH4ClO3 + 10% KNO3. Go to solution on - 18 C. Again filtered and precipitate out is again KNO3. Solution content NH4ClO3 99% + 1%
KNO3 OK. heated on 40C , respectively evaporate H2O + ethanol. Dry crystals NH4ClO3 arises ? You can added values in grams? Thanks.
|
1,0 mol of KClO3 is 122,55g , i used 10% of 1,0 mol or 1/10 mol , so i used 12,5g
1,0 mol of NH4NO3 is 80,043g , i used 1/10 mol , so 8g of NH4NO3 .
The purity will depend on the amount of water in relation to ethanol, I used the least amount possible of water and too much ethanol, spend too much
ethanol in this procedure, I think that is very better to use NaClO3 or ammonium sulfate instead of ammonium nitrate .. Dr.liptakov is you ?? What
do you want mmake with ammonium chlorate ??
wait 1 hr because is need for reaction to happen , of way correct , this reaction KClO3 + NH4NO3 is very slow .. prefer NaClO3
[Edited on 8-3-2017 by edsonmartins113]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
No need to wait 1 hour after your KClO3 and NH4NO3 are dissolved in water. KClO3 and NH4NO3 are fully ionized in water, when dissolved. You just have
free ions NH4(+), K(+), ClO3(-) and NO3(-) and these do not react at all in solution. On adding the ethanol, apparently the K(+) ions and NO3(-) ions
first combine to the solid.
After adding the ethanol it may be good to wait some time, allowing KNO3 to settle from solution.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by woelen  | No need to wait 1 hour after your KClO3 and NH4NO3 are dissolved in water. KClO3 and NH4NO3 are fully ionized in water, when dissolved. You just have
free ions NH4(+), K(+), ClO3(-) and NO3(-) and these do not react at all in solution. On adding the ethanol, apparently the K(+) ions and NO3(-) ions
first combine to the solid.
After adding the ethanol it may be good to wait some time, allowing KNO3 to settle from solution. |
Following the tables and graphic I have provided...
Into water KClO3 is the least soluble and into ethanol it seems to be KNO3 (but only in absolute ethanol) once water is present...KNO3 becomes more
soluble than KClO3 (see 50% and 90% ethanol into lower part of first document)
Into ethanol all the protagonists are less soluble than into water.
So I guess there is coprecipitation by order of priviledge KClO3 >NH4ClO3> KNO3 > NH4NO3...
So the solid that is disgarded is not mainly KNO3 as expected but truely KClO3...leaving behind a majority of NH4NO3 and traces of KNO3 and NH4ClO3
into solution.
No doubt the remaining solution when concentrated will leave NH4NO3 sensitized by KNO3 and NH4ClO3 traces...so when mixed with Al powder like the OP
did...it makes a sensitized Ammonal (NH4NO3/Al) and if actuated by a detonator like HMTD (the OP did it) of course it will detonate between 5km/s
> x > 3km/s...
I propose that the disgarded solid be tested for chlorate content by mixing dry with suggar and adding a few drops of concentrated H2SO4
--> if K chlorate it will bubble slightly yellow-green and burst into fierce flame;
--> if KNO3 it will emit orange-brown NxOy fumes and bubbles).
Into water NH4ClO3 has about the same solubility as KNO3...and it must be also much less soluble when ethanol is added...as proven by the NH4Cl,
NH4ClO4 related compounds...solubility of NH4ClO3 into water or into ethanol (or mix thereof) must lay inbetween those of NH4Cl and NH4ClO4...
Side note:
It is to be noted that into the tables one may see that for the case of NaCl/NaClO3...the use of aceton is preconized to effect separation because
NaCl is quasi totally unsoluble into aceton while NaClO3 is very soluble in it and NaClO4 even more...
Here it seems it is also the case with absolute ethanol that is quite a good solvent for that specific chlorate and perchlorate but not a good one for
NaCl...
This is very interesting for all the old weed killer (60% NaClO3/40% NaCl) bags I own or for the purification from electrolysis chlorate cell from
chloride...
[Edited on 9-3-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
edsonmartins113
Harmless

Posts: 15
Registered: 5-3-2017
Member Is Offline
Mood: No Mood
|
|
If used ammonium sulfate (NH4)2SO4 instead ammonium nitrate NH4NO3 can be better , with ammonium sulfate is not necessary to use ethanol 96%+ , in
this case use 2.0 mols of KClO3 and 1.0 of ammonium sulfate to 2.0 mol of NH4ClO3 and 1.0 mol of K2SO4 , the potassium sulfate is much less soluble
than ammonium chlorate in water , in this way it is much cheaper and easier to purify, and the best without using ethanol that is expensive 
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@PHILOU Zrealone: Interesting info. With that added info I indeed have to agree and I am now also inclined to think that the OP tested with mainly
NH4NO3, which contains a little chlorate as well. On heating that product almost certainly will decompose quickly and it indeed can react
energetically and made much more sensitive than NH4NO3 by its small chlorate content.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
1,0 mol of KClO3 is 122,55g , i used 10% of 1,0 mol or 1/10 mol , so i used 12,5g
1,0 mol of NH4NO3 is 80,043g , i used 1/10 mol , so 8g of NH4NO3 .
OK..thanks. First part of question we have. 12,5 + 8g. Second part of question: And water is how much. 100g? 50g? 10g ? Water (dissolving process) in
Lab. temerature 15 - 25 C? And ethanol into water for precipitate : adding 10g? 90g? or 100g ? Thanks....Yes Dr. Liptakov..... .... ....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
What a criminal waste of precious ethanol !   
Exact science is a figment of imagination.......
|
|
|
| Pages:
1
2 |