morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
Aurin
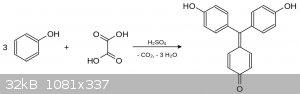
Didn´t find anywhere exact procedure for synthesis of aurin from phenol and oxalic acid, can somebody help? Is evolution of carbon monooxide from
oxalic acid problem?
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The usual condensing agent is anhydrous zinc chloride, See Richter vol 2 p593. He refers to the use of phenol, formic acid and zinc chloride for the
preparation of aurin but oxalic acid will probably work too with the evolution of carbon dioxide. If you want an actual reference try looking in one
of the older chemical disctionarys such as Thorpes.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Aurin wikipedia
also good to check other languages like German or Dutch.
Into the german one they give reference 6:
S. Hauptmann, J. Gräfe, H. Remane:
Lehrbuch der organischen Chemie, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig 1980, S. 694.
[Edited on 24-1-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I was curious about OP's relatively simple synthesis of aurin. I found the following synthesis in the Journal of the Chemical Society, Transactions.
Redistilled phenol (1,000 grams) was heated with 500 grams of concentrated sulphuric acid and 650 grams of dehydrated oxalic acid in an open flask at
135-145'. Gas evolution ceased after twenty-four hours, and the hot, dark red, viscous mass was poured into a large quantity of cold water.
The pasty deposit was repeatedly digested with several litres of hot water, the dye gradually becoming more viscous in the hot and more brittle in the
cold through the elimination of unchanged phenol and soluble by-products. The strongly coloured wash waters were poured into hot, and the red solid
deposited by the several wash waters cooling gradually diminished to a negligible amount. The solid deposited by the first wash water amounted to 100
grams. The final wash water was pale yellow and was free from sulphuric acid. The washed dye was freed from water as much as possible by squeezing it
while soft and warm. On cooling, it formed a fairly hard mass with a green lustre. Weight, 320-550 grams.
|
|
|