| Pages:
1
..
5
6
7
8
9
..
25 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Interesting. So you got fourier transform infrared spectoscopy available?
You know what you should do with this? Analyse the putative DPPP, the thread of which you surely must have noticed!
[Edited on 8-6-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
The final yield of HS from the chlorourea process was 9.62 grams. My guess was high as the crystals must have contained more water than I had guessed,
but still a good quantity.
Here are a few pictures. The pan on the right contains high grade HS from a known source. The pan on the left is the material from chlorourea process.

|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
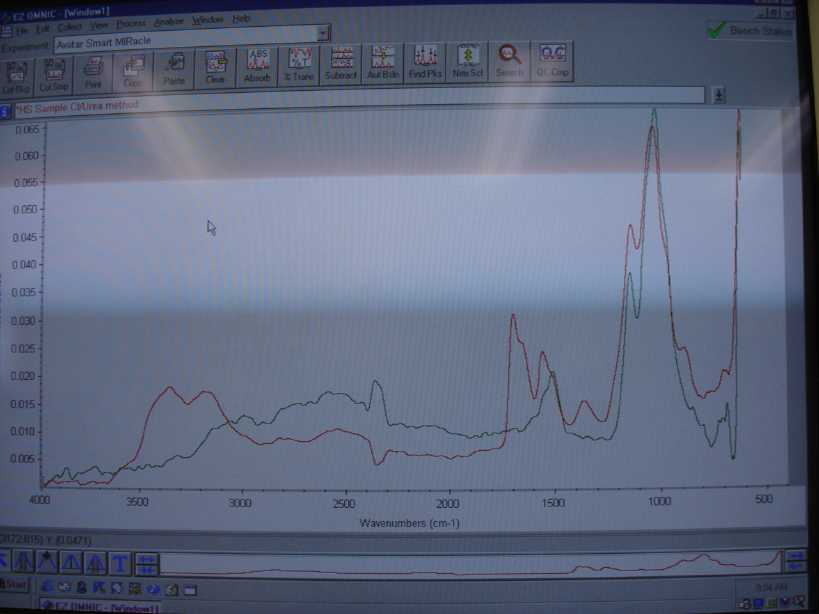
The spectra in red is the material from the chlorourea process and the one in green is from my known source which is at least tech grade or better.
Unfortunately my libraries did not contain a commercial spectra of HS so we all will have to compare by eye. The peaks under 3200 and 3400 are
probably from residual H2SO4. I need to recrystalize and run it again but it looks like a good match from my experience.
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Sorry about the multiple posts but I can't seem to figure out how to post more than one pic at a time with my old Mac.
Here are the starting materials for my HS from chlorourea experiments.

|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Where I got my known good hydrazine sulfate from.

|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Good, work, ordenblitz!
But why was your yield so much higher than mine?
I suspect that you added more chlorine, since my chlorourea solution was only faintly yellow and your solution strong yellow.
Be careful though, NCl3 has exactly this intense yellow color...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The modification of the chlorourea process for semicarbazide as the end product should also be interesting .
There has been mention of the possibility
of using semicarbazide as a precursor to azides , but I know of no references nor details concerning the reaction .
Earlier in the thread there was mention about the possible danger of nitrogen trichloride being a byproduct with the chlorourea process . It was
mentioned in the pdf regarding the use of TCCA as an oxidant on page 2 , that it is possible for nitrogen trichloride to form in strongly acidic
solutions of TCCA , so this may need to be considered when the source of chlorine is TCCA . Whether the formation of nitrogen trichloride is
temperature dependant or not I do not know for certain . It would probably be a hazard avoided simply by conducting the chlorine generation at warmer
temperatures at which any NCl3 formation or accumulation would not be favored , and evolution of Cl2 would be
assured . Curiously this is likely one of those reactions where heating is the essential guarantor of safety , and cooling would not be a good idea
for the TCCA based " chlorine generator " 
A parallel is likely true for the chlorourea formation . Keeping the temperature slightly warm , maybe 25 C for example ,
would be safer than say using an ice-salt
cooling for the chlorination of the urea .
[Edited on 14-6-2005 by Rosco Bodine]
|
|
|
hannibal
Harmless

Posts: 6
Registered: 24-5-2005
Location: Carthage
Member Is Offline
Mood: reasonable
|
|
hydrazine synthesis continued
sorry, for starting this new thread......somehow i could not post my reply on the previous thread on "hydrazine synthesis" , apparently it
has been closed.
in one of the previous post by NickF he proposes that use of calcium hypochlorite increases the total conversion of hydrazine.
I am quoting him
"I'd recommend using urea instead of ammonia for making hydrazine. It means you don't get a house full of ammonia, especially if you
can't get very good ventillation!
Check out the Hive for a synth from urea. I tried it, using fertiliser grade urea, <5% NaOCl household bleach, gelatine, drain-cleaner NaOH
granules and tap water. The yield was just over 15%, while this is not very good it is a very cheap method. The yield is improved greatly by using
Ca(OCl)2 instead of NaOCl, because of the Ca(OH)2 present. Adding Ca(OH)2 to a synthesis using NaOCl also increases efficiency. Using distilled water
helps by reducing the ammount of transition metal ions, and using pure urea would also be a great help. The text I followed reported a 75% yield based
on urea!
To make azides, it's probably best to first make a solution of hydrazine nitrate, by mixing strong solutions of hydrazine sulphate and calcium
nitrate, and then filtering off the CaSO4 ppte. Otherwise you might end up with a lot of ppted PbSO4/Ag2SO4 in your product, depending on what you are
trying to make.
I wanted to make semicarbazide for NTO and azides, but never got a very good yield from any of the methods I tried. For making azides, it's MUCH
easier to use hydrazine (and semicarbazide is not very much less toxic than hydrazine anyway). It would be nice to be able to make NTO, but because of
the trouble of having to make the semicarbazide it's better to use another HE." ---- by NickF
If this is true then why use of sodium hypochlorite is recommended,although it is more expensive than calcium hypochlorite(because calcium hydroxide
is cheaper than sodium hydroxide)......if we use calcium hypochlorite there is one more advantage that i see calcium carbonate formed in the reaction
can be easily separated
from the hydrazine solution(because solubility of CaCO3 is less),this decreases the amount of sulphuric acid needed in the precipitation of hydrazine
sulphate.
2Ca(OH)2+2NH2CONH2 +Ca(OCl)2---->2N2H4 + 2CaCO3 +CaCl2 +2H2O
Although the hydrazine sulphate that we get will be slightly more impure because of calcium sulphate precipitation in this case.
one more point why in this case a bivalent ion (Ca2+) does not catalyse the oxidation of hydrazine....if it does then why hydrazine yields are high.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by hannibal
If this is true |
* If * is a very big word in this entire matter . Suppose that the use of Ca hypochlorite is not superior , then what
good are the rest of the rational analyses ? No one has described the
details of a step by step method using
Ca hypochlorite with comparisons of the
yield with the sodium hypochlorite method , which is well proven and gives good yields . Ca hypochlorite is NOT cheaper than sodium hypochlorite ,
except
for those industrial scenarios where the
hypochlorite solutions are made on site for
immediate use . When carried to the point of making stable products for distribution and sale , the sodium hypochlorite solutions are cheaper to
produce . Compare the OTC price of a gallon of 10% sodium hypochlorite for $1.50 , with an amount of granular HTH having the same chlorine content
and you'll see very quickly the " dry chlorine "
product is several times more expensive .
| Quote: | then why use of sodium hypochlorite is recommended,although it is more expensive than calcium hypochlorite(because calcium hydroxide is cheaper than
sodium hydroxide)......if we use calcium hypochlorite there is one more advantage that i see calcium carbonate formed in the reaction can be easily
separated
from the hydrazine solution(because solubility of CaCO3 is less),this decreases the amount of sulphuric acid needed in the precipitation of hydrazine
sulphate.
2Ca(OH)2+2NH2CONH2 +Ca(OCl)2---->2N2H4 + 2CaCO3 +CaCl2 +2H2O
Although the hydrazine sulphate that we get will be slightly more impure because of calcium sulphate precipitation in this case.
one more point why in this case a bivalent ion (Ca2+) does not catalyse the oxidation of hydrazine....if it does then why hydrazine yields are high.
|
Anyone is welcome to test variants and chart the results of what improvements are
* proven * by alternate hypochlorites ,
mixtures of hypochlorites , and modified conditions for the reaction which * prove *
useful . But since it is known for certain that the urea process is sensitive to small variables , then there is no basis for any conclusions about
what is better in the way of changes to the known process ,
before actually testing and retesting the modification to make certain it has validity . Knowing how sensitive the reaction is to small variables is
exactly why I performed a half dozen experiments for confirmation , before having the boldness to declare that use
of hydrochloric acid for the initial neutralization , and then following that with sulfuric acid for securing precipitation of the sulfate , was a *
superior * method . You see there is a huge *inertia* associated with textbook methods which have been in practice for a hundred years , and any
maverick suggesting changes should be sure they aren't calling a press conference in Utah to report their success at cold fusion using a method
nobody else on earth can reproduce 
[Edited on 17-6-2005 by Rosco Bodine]
|
|
|
hannibal
Harmless

Posts: 6
Registered: 24-5-2005
Location: Carthage
Member Is Offline
Mood: reasonable
|
|
after going through the entire discussion i still have some questions regarding synthesis of hydrazine from urea and sodium hypochlorite process ----
1.why are we adding excess NaOH in the sodium hypochlorite solution before adding urea into it and then take pain to again cool it to keep it
stable.....a better way would be to add NaOH along with urea into hypochlorite.This will also decrease the amount of heat that we need to supply
during the hydrazine formation.
2.why are we adding urea to hypochlorite and not hypochlorite to urea.
i think for somewhat larger batches adding of hypochlorite to urea would be better.....has someone tried it by adding hypochlorite to urea???????
3.we all are measuring the yield of hydrazine sulphate and not actual hydrazine formed (some amount remain dissolved in the solution), this method
will give lower conversion and also conversions that cannot be compared as we all are dealing with different volumes of solutions.There are also some
other complications like sodium sulphate precipitation along with hydrazine sulphate........i think there is a method for quantitative analysis of
hydrazine by titrating it with iodine although i dont know the exact equations involved but know its pretty simple, the exact method for titration
should definitely be in "hydrazine and its derivatives".Please post this method if anyone gets it
[Edited on 18-6-2005 by hannibal]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
What does it matter for anything other than anylitical chemistry the 'real' yield of hydrazine formed? What matters to us and others in
practical chemistry is the ammount of product that can be isolated from the reaction mix. Besides, using HS solubility we can figure out aproximatly
how much is still in the solution.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@ hannibal
There is nothing arbitrary about the methods which have been worked out for the synthesis of hydrazine , by methods which are practical for small
scale production . The literature including the patents will give you the reactions which apply , and you can justify the proportions of reactants
and their order of addition , and conditions of temperature
and concentration required , by experiments and comparisons of your
observations and results . An understanding of the chemistry and the properties of the materials used in the reaction , would set aside some of your
questions before they are asked , such as why the base isn't mixed with urea , which would decompose the urea . But you can try it and see and
also see whether that improves the production of hydrazine . Also reversing the order of addition you can try and see what happens . What we are
trying to do is to pin down the details and refinements upon methods which are known to work well , not so much to explore every possible variation
which imagination can provide , including those which seem contrary to theory . It requires enough time and work simply doing the experiments which
follow from the logic which can be best defended . So there is little enthusiasm for experiments which look for the unexpected anomaly rarely found
when a synthesis is deliberately run in a direction against theory , simply for satisfying curiosity as to what will be the result . Usually what
happens is you either get none of the intended product at all , or a mixed product with all the possible byproducts
which may occur in a mixture which hasn't been deliberately steered to favor one specific target product from controlled reactions .
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Hydrazine From Urea
Hannibal, the production of hydrazine from urea is more efficient, and less smelly, than
from ammonia. As far as your newer thread on this subject, The Bird has the final word !
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
hannibal
Harmless

Posts: 6
Registered: 24-5-2005
Location: Carthage
Member Is Offline
Mood: reasonable
|
|
rosco i dont think i have written anything illogical ..... regarding mixing of urea and the base issue let me make myself clear ..... i am not mixing
sodium hydroxide in urea and then adding it to hypochlorite instead i am adding urea and sodium hydroxide separately but simultaneously in the
hypochlorite.pls tell me if i have made myself clear this time.
anyways i am planning to do some experiment somewhere in the mid of next month to see the results myself...any suggestions???????
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
hannibal ,
The suggestion I would emphasize is that the first time you make hydrazine , in order to see exactly what sort of reaction dynamic which occurs pretty
rapidly and in a narrow range of conditions , first duplicate one of the methods that have already been worked out and proven . You will then have an
insight into some factors which make these labscale batch processes necessary to be done the way they are done , to simplify things and to get a
decent yield by a lab method that is
convenient . Many of the things which could be done in an industrial process are
not easily adapted to a batch process or
produce so little increase in yield as to not be worth the trouble . On an industrial scale with continuous separate feeds of separate precursors ,
where controlled flow rates and reaction temperatures , residence times and other
parameters can be controlled , it is easy enough to explore many variations which can tweak the reaction and get a few per cent increase yields . It
is much more difficult to implement manageable changes to a vat process which is already
optimized for a lab scale . It is even more difficult to do that for a reaction that you don't appreciate is more complicated than
just a linear reaction like A+B+C=D . Hydrazine is actually a competing reaction with a lot of other possible reactions , and if you don't hit
the target conditions just right , then you won't get a good yield of hydrazine . Concentration of reactants is a factor which makes the
solubility of the precursors a very pertinent concern , so the low solubility of
CaOH does not fit the right thinking that you need a very soluble base for a concentrated reaction mixture , aside from the extra steps introduced by
having to separate calcium byproduct precipitates . When you have experience with the reaction that works , then you will understand that some of the
things you suggest actually aren't logical . Do some experiments to prove that if you need the proof . When you get better yields than 65% of
dried crystals of pure hydrazine sulfate , then describe the details of what works better than what we already know and there won't be any
speculation about what is an improvement
* after * the details are known . Debating the details in advance of the finding is premature .
I can make kilos of hydrazine if I want it , while you are trying to analyze why your failed experiments didn't work . There's the point .
As for using the heat of dilution of the NaOH to be an enhancement of the exotherm for the production of the hydrazine , well that would make sense if
the reaction needed the boost . Simply varying the initiation temperature upwards a few degrees will give you all the boost you can handle ,
especially for larger batch sizes . So you will never need the added exotherm from such a strategy . Also there would be the difficulty introduced
of getting a consistent mixture , before the foaming complicates any further addition , which sometimes occurs almost instantly , especially at warmer
temperatures for the start of the reaction .
[Edited on 20-6-2005 by Rosco Bodine]
|
|
|
hannibal
Harmless

Posts: 6
Registered: 24-5-2005
Location: Carthage
Member Is Offline
Mood: reasonable
|
|
rosco let us talk ablout these things after i complete the experiments and believe me my aim would not be to prove u wrong but to give an improved
synthesis....can anyone tell me about the reaction of hydrazine with iodine in sodium bicarbonate...may be madhatter u can tell from "hydrazine
and its derivatives"
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
hannibal ,
The biggest potential improvement I see possible in terms of increasing the yield of hydrazine per unit volume of reaction mixture , is in combining
the chlorourea method with the urea-hypochlorite method as I mentioned earlier in the thread . Concurrent isolation of the hydrazine as a hydrazone
intermediate might be another possible enhancement ,
but whether these things are cost effective in terms of the added steps and reagents , over the straightforward single methods is unlikely . It is
probably better just to increase the batch size . That's another real consideration for reactions that are producing yields at 65% , where you
may be able to improve that to 75% or 80% , but the tradeoff is that the added work or cost of added reagents , or both combined , makes it easier
and more economical just to increase the batch size for the lower yielding method , and be content that
65% is " good enough " , if you follow the rationale .
[Edited on 21-6-2005 by Rosco Bodine]
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Hydrazine And Its Derivatives - 1st Edition
Bear in mind that my book is 1st Edition and published in its 1st year - 1984. The 2nd Edition,
which I can't find at reasonable price for the moment, is twice as long - even in the synthesis
section.
Hannibal, there is no mention of iodine or sodium bicarbonate being used in the urea process -
at least not in the 1st Edition. I don't know about the 2nd Edition because I haven't
seen it - yet ! This edition states the maximum efficiency of the urea process is 64%. Maybe there's
been improvements discussed in the 2nd Edition, so Roscoe it sounds like your right on track.
Hannibal, don't be discouraged. Do some testing, and if you can improve the yields, we'll all
be looking forward to it !
BTW, I haven't been able to do much scanning lately. I'm setting up an FTP server with
200 GB capacity and I'm still working on the configuration. The 1st Edition will be the 1st book
I put there.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Analysis
Hannibal, sorry I misread your post about iodine and bicarbonate. I belive this is the information
from page 415 of Hydrazine And Its Derivatives, 1st Edition, 1984, that you're referring to:
" Hydrazine can be titrated directly with standard iodine solution if a
buffer is added to keep the pH between 7.0 and 7.4. The last drops of iodine
must be added slowly, and several seconds must be allowed for complete reac-
tion. At a pH lower than 7, the reaction is quantitative, but very slow. If
the pH increases above 7.5, hypoiodite formation may cause low results,
or hydrazine may begin to autoxidize. "
Bicarbonates, acetates, and tartrates are used as the buffers. According to the book, you can't
use too much buffer or the iodine won't be taken up - particularly true for sodium bicarbonate.
Also the last drops of iodine solution must be added very slowly. If the yellow color persists for
more than 2 minutes, the endpoint has been reached.
P.S. Still trying to get my FTP up and running. Still can't access from a remote computer.
More tweaking of the network and/or server settings necessary.
[Edited on 26-6-2005 by MadHatter]
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
mandarine
Harmless

Posts: 1
Registered: 19-7-2005
Member Is Offline
Mood: No Mood
|
|
a little help...?
just interested in ending up with hydrazine hydrate here, not a question about converting it to the sulfate.
so, I've been at this reaction for a couple weeks now. all OTC, sodium hypochlorite (30ml of 5%), 10% janitorial strength aqueous ammonia (130ml
of 10%), some gelatin (1.5 grams), and heating it to 1/3rd volume.
first of all, I'd like to clarify what on earth you end up with as soon as it has evaporated down to 1/3rd? I know there should be plenty of
Chloramine, a little hydrazine hydrate, leftover gelatin, but that should be it? the solution is very much yellow, and because Chloramine is supposed
to be yellow, I assume it's the most abundant result of the reaction.
now, I'm only interested in knowing that I can get this reaction right. I don't care about getting tons of hydrazine, I simply want to end
up with a little relatively pure hydrazine. I'd even be happy with only a couple milliliters, frankly. in fact, at this point, just knowing FOR
SURE that it's hydrazine and only having a milliliter would be REAL SWELL 

what I've been trying from this point is to add 1 volume of xylene to the resultant liquid after it's cooled down to room temp.
unfortunately I can't find any info on Chloramine's solubility in xylene, but I know that hydrazine is PARTIALLY soluble in it. good enough
for me. so I shake it in xylene, and then siphon the xylene layer into a new flask and add a couple milliliters of deionized water and shake again.
the idea, of course, being that there ought to be SOME hydrazine in that xylene, and hydrazine is far more attracted to water than xylene, so
you'd think a few milliliters of water would combine with hydrazine and become a relatively high concentration of hydrazine hydrate?
the water is siphoned out and takes on a pH of 11/11.5 (?!) (which is light brown on regular pH paper). WTF? isn't hydrazine, in its ANHYDROUS
FORM, only supposed to get up to 10 (green on pH paper)? with lower and lower pH as is becomes more dilute with water? obviously something's
going wrong somewhere, can anyone help?
now, trying to reason through this, most of the ammonia and hypochlorite would have finished reacting after the reaction mixture hit 60C, and they
surely wouldn’t be present anymore after it hits 100C (I usually heat up around 110C). so, all that we can be left with in the yellow mixture is
what I listed above, and none of those things has a pH of 11 (chloramine's pH is 9)... I'm really confused here.
how stable is hydrazine to oxygen? does it only last for a couple hours when exposed to air or something, then decomposing into ammonia/etc? I know UV
breaks it down too...
I've tried the same xylene extraction process with the liquid boiled down to 1/6th and 1/2. the final water extract still has a mysteriously high
pH.
does anyone have good ideas for testing the hydrazine?
as I know it's supposed to react a little with copper, I dripped a single drop of my supposed hydrazine hydrate onto two pennies, one modern
(only lightly coated with copper, 2.5%, the rest being zinc metal) and one from the 1950's (which is 95% copper and 5% zinc).
after sitting with a drop of the supposed hydrazine overnight, each penny turned very clearly green at the place where the liquid was, with the liquid
of course having evaporated by then.
as those results mean nothing to me, I also tried dropping a single droplet of the supposed hydrazine on an open flame 
nothing happened.
am I somehow just ending up with ammonia here or what? and if so, why?
*sigh* can anyone interpret these results? anyone have better tests for hydrazine (besides showering yourself in it and seeing if you get cancer)?
BTW, after turning the heat back on after it has foamed to evaporate down to 1/3rd, I’m always OUT OF THE HOUSE. for safety, all I do is perform the
reaction next to a big window (there’s no people and hardly any wildlife around here) with a fan blowing directly on the mouth of the reacting flask
and out the window. I also get a little dizzy from holding my breath, plugging my nose, and pressing goggles against my face whenever I have to be
near the flask (which is as rarely as possible). am I being paranoid here?
and what about Chloramine? I've searched, but can't find any info on its solubility with xylene. it's obviously very soluble in water,
as regular chlorine is being phased out in favor of Chloramine to treat public water. does anyone have a link to the chemical structure of Chloramine?
Wikipedia usually is very good about putting the structure of chemical compounds with their chemical pages, but not for chloramine.
maybe someone has a suggestion for a fool proof method to extract hydrazine from this yellow liquid? and speaking of yellow liquid, where can one go
to get OTC urea? I assume fasting for a day, drinking lots of water and peeing onto a hypochlorite isn't an advised method of getting hydrazine

and here's another question: would adding some gypsum salts, epsom salts, or other drying agents along with the gelatin be a good idea for ending
up with less water and more hydrazine? probably not?
and here’s on FINAL question (sorry for all the questions!). I’ve heard that only anhydrous hydrazine can react with an amine to produce an azide
(when considering only the hydrazine family anyway). is this true? can hydrazine hydrate work to produce an azide from an amine too? what about if
it's very dilute?
P.S. all non-OTC suggestions, while appearing very interesting to me, will not help anything 
[Edited on 20-7-2005 by mandarine]
[Edited on 20-7-2005 by mandarine]
[Edited on 20-7-2005 by mandarine]
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Hydrazine is very reactive toward oxygen. In fact, one of its main industrial uses is as an oxygen scavenger in boilers because it reacts so well.
I'm not sure about amines, but nitrites are the standard reagents we use to make azide around here.
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Wow.... lots of questions.... I'll answer what I can. First off, you could just attempt to distill hydrazine hydrate from your
mixture for making hydrazine in the first place paying careful attention to the fractions and collecting at the boiling point of the azeotrope, I have
read of this being done. Also note that hydrazine is recomended to be distilled under hydrogen in a copper or silver apparatus, it will react with
glass to the best of my knowledge (though my distillation attempts of hydrazine hydrate show no attack on the glass) also note that ground glass with
hydrazine on it can explode, the high surface area working catalytically with oxygen in the air. Hydrazine will react with the oxygen in the air
anyway under its own power with little provolking.
Now, you are trying to see if you have hydrazine by checking the pH of the solution you get from extracting with xylene and adding water, hydrazine is
actually classified as a somewhat strong base, anhydrous hydrazine might have a lower pH then the hydrazine hydrate, same methodology that ~75%
sulfuric acid has more hydronium ions then 100% sulfuric acid. So the pH method is not a good test for hydrazine.
As for tests for hydrazine there are little pads of paper that you can put water on containing hydrazine which will detect in the ppm but are
otherwise useless, you could test it's reducing power using the silver mirror test or something similar but there are other qualitative tests for
hydrazine, a few are in this thread if you read it carefully.
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Mandarine,
IMHO the best way, is to proceed as directed and convert your product to hydrazine sulfate. You will then be able to determine easily how much
hydrazine you actually made. If you go back on the thread you will find directions on freebasing with NaOH back to hydrazine. Many in the explosive
industry purchase their hydrazine as the sulfate and recover as needed. It's the safest and best way to store NH2NH2.
[Edited on 21-7-2005 by ordenblitz]
|
|
|
nikcorbet
Harmless

Posts: 2
Registered: 9-9-2005
Member Is Offline
Mood: 999
|
|
Hydrazine
What MP of hydrazine sulfate you get by Urea process . can any one tell actually it should be 254 Deg
C but its not that after crystln twice 
[Edited on 9-9-2005 by nikcorbet]
999
|
|
|
redneck
Harmless

Posts: 12
Registered: 26-9-2004
Member Is Offline
Mood: No Mood
|
|
Has anybody any expierience with this synthesis?
Working up synthesis solutions obtained in production of hydrazine , United States Patent 4005179
Abstract: In the production of hydrazine wherein aqueous ammonia is oxidized in the presence of a ketone to form an aqueous solution containing at
least one of a hydrazone and a ketazine along with ammonia, the hydrazone and ketazine are concentrated and the hydrazone and ketazine are
subsequently hydrolyzed to hydrazine and ketone, the improvement which comprises effecting the concentration of the hydrazone and ketazine by
extracting the aqueous solution with a substantially water-immiscible organic solvent whereby the hydrazone and ketazine preferentially enter the
water-immiscible solvent, and separating the water-immiscible solvent extract from the aqueous solution. The organic solvent is preferably a higher
alcohol, a chlorinated hydrocarbon, benzene or a substitution product thereof. The organic solvent extract, in one or more stages, and optionally with
addition of ketone, can be re-extracted with water, hydrolyzed with aqueous acid or subjected to distillation to separate the hydrazine values from
the organic solvent.
see:
http://freepatentsonline.com/4005179.html
|
|
|
| Pages:
1
..
5
6
7
8
9
..
25 |