| Pages:
1
2
3 |
szuko03
Hazard to Others
  
Posts: 188
Registered: 3-4-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
Wow I had no idea they made anti static brushes with radioactive elements. It just seems like a waste for something so expensive and rare in a sense.
[Edited on 28-4-2015 by szuko03]
Chemistry is a natural drive, not an interest.
|
|
|
veganalchemist
Harmless

Posts: 30
Registered: 3-8-2010
Location: UK
Member Is Offline
Mood: No Mood
|
|
Here are a few pictures of my Periodic Table.
I finally got my EPP licemce from the Home Office (UK).
 
I normally have it covered with a perspex sheet.
The Silver coin is a 2014 UK quarter ounce siver coin struck from the silver recovered from the SS Gairsoppa. Got it from the Royal Mint. You get a
really interesting booklet and DVD about the salvage operation.
The wreck is at 4 700 m (The Titanic is at 3 700 m).
The gold bar is a 5 g 999.9 fine gold bar, also from The Royal Mint in Wales.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Looks great! Nice work.
I'm curious what you have for your radioactive samples: Po, At, Rn, Fr, Ra, Ac, Pa, Np, and particularly Pu?
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
That is a gorgeous display and an even more gorgeous collection. Well done.
What did you use for F?
|
|
|
veganalchemist
Harmless

Posts: 30
Registered: 3-8-2010
Location: UK
Member Is Offline
Mood: No Mood
|
|
I need to replace some of the reactive metals in oil as they are badly corroded.
Po is from an antistatic brush.
At, Fr and Pa are from pitchblend.
Rn is from some thorium nitrite.
Ra is a luminouse watch hand.
The F sample is a 33% F sample in nitrogen. Prabbably escaped by now. Given to me as a Christmas pressent from a good friend.
And finally the Pu is a sample of Trinitite.
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Here is my effort at a periodic table with a few notes.
1. I deliberately stopped at Bismuth as radioactives are impossible to get in elemental form in the UK.
2. There are some gaps. I am trying to find a supplier of small amounts of rubidium (0.5 to 1 gram) as the current ebay offerings of 20mg are too
small.
3. There are more gaps around the noble metals (Osmium etc as they are very expensive)
4. For the Noble gases I have used a photo of the gas discharge glow for each.
5. For Fluorine I use a Fluorite crystal which can be illuminated with UV as I'm never going to get hold of visible amounts of fluorine.
6. The other colourless gases ??.
 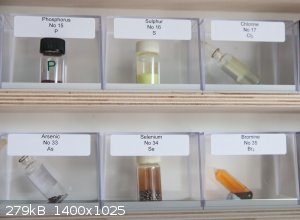 
If you're not part of the solution, you're part of the precipitate.
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
very nice guys...
Beginning construction of periodic table display
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
I do not have as nice a display as some of these, but for F, I do have a sample of antozonite, which contains small amounts of elemental F. It does
not look as interesting as the usual fluorite crystals, but I still like it.
https://en.wikipedia.org/wiki/Antozonite
http://www.nature.com/news/stinky-rocks-hide-earth-s-only-ha...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Me too! A commenter on one of my videos mentioned it, and after searching around eBay for a long while I finally found a sample. I was very excited to
learn about a way to have elemental fluorine for my collection!

They are the little black cubes (actually very dark purple), growing on a quartz crystal.
|
|
|
CouchHatter
Hazard to Others
  
Posts: 152
Registered: 28-10-2017
Location: Oklahoma
Member Is Offline
Mood: 76 elements taken!
|
|
my periodic display
I have been imagining this for a couple of years. Finally put in the work to build it - now the collecting can begin in earnest!
I skipped on the lights, only because I'd already spent as much time as I cared to building it. It took 20+ hours of marking and cutting, routing and
sanding, painting and folding to realize this much of it. And I am starting school so I wanted to have this up on the wall not on the shop floor.
Now to tidy up my neglected lab...

|
|
|
| Pages:
1
2
3 |