| Pages:
1
..
46
47
48
49
50
..
81 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
Are there any stable binary liquid mixes that deflagerate (or preferably detonate) at the same speed or faster as flash powder when ignited by a fuse?
|
Stability is in the eye of the beholder... How long, what storage conditions?
Yes, plenty of room temperature and various cryogenic temperature possibilities come to mind. Google for Sprengel explosives. Plus, check for the
Oxyliquits, liquid O2 with just about anything flammable.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by nitro-genes  | NM with hydrazine may form some hydrazinium nitromethanate, methazonate or reaction products thereof, which reportedly are very reactive/explosive. If
they are soluble enough in the NM a fuse sensitive mix might result (unlikely though) Couldn't find much about the products formed of mixing NM and
hydrazine, which may be interesting in itself. Organo metallic compounds might also produce sensitive liquid mixtures with oxidizer, though since many
are pyroforic/hypergolic very impractical probably.
[Edited on 24-10-2016 by nitro-genes] |
IMO nitromethane would be reduced by hydrazine to methylamine
|
|
|
JefLeb
Harmless

Posts: 4
Registered: 21-10-2016
Member Is Offline
Mood: No Mood
|
|
Thank you Tin man.
So the layout would be as in the following figure?
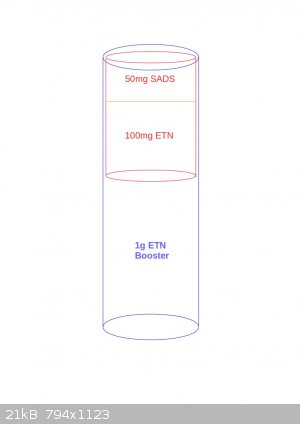
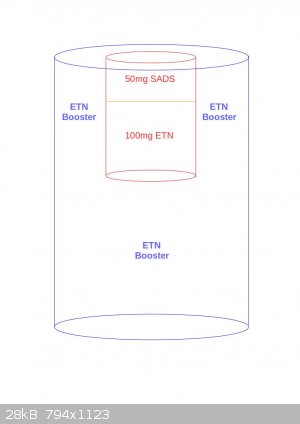
Or maybe this way?
[Edited on 25-10-2016 by JefLeb]
|
|
|
yobbo II
National Hazard
   
Posts: 778
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
The was a mixture used during WWI or was it II that consisted of carbon disulphite and something (Nitrious oxide). They were placed into a two
compartement tank and when the tank fell off the aeroplane there was a propeller on the tank which opened a door between (agitated too) the
substanced mixed and when it hit the ground it went kaboom.
|
|
|
Bert
|
Threads Merged
25-10-2016 at 07:25 |
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  |
| Quote: |
Are there any stable binary liquid mixes that deflagerate (or preferably detonate) at the same speed or faster as flash powder when ignited by a fuse?
|
Stability is in the eye of the beholder... How long, what storage conditions?
Yes, plenty of room temperature and various cryogenic temperature possibilities come to mind. Google for Sprengel explosives. Plus, check for the
Oxyliquits, liquid O2 with just about anything flammable. |
Hmm. Storage stability, I would hope we would be looking at, at least a few days in varying temps. All of the options I hear seem to have extremely
corrosive or toxic liquids. Bummer
|
|
|
Tin man
Harmless

Posts: 34
Registered: 12-9-2016
Member Is Offline
Mood: Gruntled
|
|
Jefleb, the second one is about right. The proportions are a bit off, I was thinking everything would be a bit narrower, but it should still work.
Happy blasting!
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Initially some nitronate salts are formed (US 2963507 A). Nitromethane sensitized by hydrazine is described as being reasonably stable. Also see: US
3980510 A, although the formed compunds are not tested. It would be hilarious if the remaining sensitizer after prolonged contact with hydrazine is
actually methylamine though. 
[Edited on 25-10-2016 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Methylamine is the result when Nitromethane is in isopropanol reduced with aluminum amalgam (aluminum isopropoxide).
That reduction is instant and quantitative IIRC. With hydrazine as the reductant it appears to preferentially form an adduct with the nitroparaffin
according to the patent.
[Edited on 10/26/2016 by Rosco Bodine]
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JefLeb  | Thank you Tin man.
So the layout would be as in the following figure?
Or maybe this way?
[Edited on 25-10-2016 by JefLeb] |
The lower booster should be cast, and the cap should be 1gram pressed ETN should give you good result
|
|
|
Tin man
Harmless

Posts: 34
Registered: 12-9-2016
Member Is Offline
Mood: Gruntled
|
|
XeonTheMGpony: that would make a great blasting cap, but isn't casting large amounts ETN a little dangerous for someone new to energetic?
|
|
|
JefLeb
Harmless

Posts: 4
Registered: 21-10-2016
Member Is Offline
Mood: No Mood
|
|
I'm sorry for the late, I've been very busy.
Thanks a lot for all your advices Tin man and XeonTheMGPony.
I've tried depicting the correct version of the detonator taking into account your recommendations. Do you think this one is correct? Or maybe I
should put 1g ETN in the red cap instead of 100mg ETN? (I'm not sure whether or not XeonTheMGPony meant that)
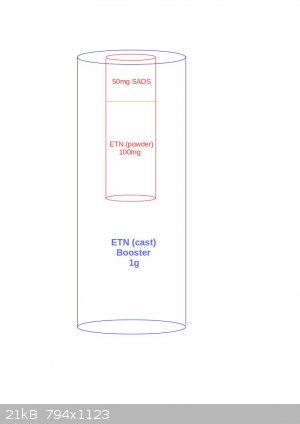
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Just be sure to use a tube larger than the critical diameter for cast EFN. It was6 mm I think. Otherwise you will just end up with an under powered
cap.
|
|
|
ill behaviour
Harmless

Posts: 8
Registered: 18-5-2016
Member Is Offline
Mood: No Mood
|
|
I have a couple of questions to ask. If anyone can point me in the right direction it would be appreciated.
When using other nitrate salts in NM compositions when AN is not possible, for example potassium nitrate, as I understand it the gas production is
reduced but is the VOD basically the same?
Can oxygen balance be achieved by simply adding or subtracting the known + or - oxygen balance, I thought I read that NM is -39.3 per 1g and PN is
+47.5 per 1g is this correct.
When making a NM composition for maximum power would the nitrate salt or amine sensitized variety be preferred. The NM/nitrate would have a lot less
NM content so I really confused about whether the NM confers its explosive properties to the entire weight of the nitrate or just takes the oxygen.
|
|
|
Thraxx
Hazard to Self
 
Posts: 71
Registered: 15-10-2016
Member Is Offline
Mood: No Mood
|
|
Hi,I would like to ask you,what you mean about the idea-
The Earl Warwicks Silly Putty, like plastificator.
Silly Putty invented 1943,composition of 65% dimethyl siloxane (hydroxy-terminated polymers with boric acid), 17% silica (crystalline
quartz), 9% Thixatrol ST (castor oil derivative), 4% polydimethylsiloxane, 1% decamethyl cyclopentasiloxane, 1% glycerine, and 1% titanium dioxide.
The idea to use silly putty like plastificator was publlished by amateur author Exxtec(Pyroforum),which described how to make a detonable
composition Silly Putty(28%)+Petn-RDX with spec.grav. 1,42 g/cm3.
One kind of this putty is full of Aluminium powder.And I think,that this kind of putty filled with Petn will under pressure of detonation
react between SiO2 + Al==Al2O3 + Si.
Can you tell me please,1)if such composition could be good? 2) if there will be DINGU on the place of Petn/Rdx,then could this a bit
acid putty be a stabilising factor for the Dingu?
Thank you Thraxx
[Edited on 15-10-2016 by Thraxx]
[Edited on 15-10-2016 by Thraxx]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ill behaviour  | I have a couple of questions to ask. If anyone can point me in the right direction it would be appreciated.
When using other nitrate salts in NM compositions when AN is not possible, for example potassium nitrate, as I understand it the gas production is
reduced but is the VOD basically the same?
Can oxygen balance be achieved by simply adding or subtracting the known + or - oxygen balance, I thought I read that NM is -39.3 per 1g and PN is
+47.5 per 1g is this correct.
When making a NM composition for maximum power would the nitrate salt or amine sensitized variety be preferred. The NM/nitrate would have a lot less
NM content so I really confused about whether the NM confers its explosive properties to the entire weight of the nitrate or just takes the oxygen.
|
The OB is defined as a % and as such it is based on 100g not on 1!
The rule of additivity is not valid here because you are playing with % and to add a/100g + b/100g means a+b on a total of 200g!
Thus into a 1/1 weight basis mix.
Your NM+KNO3 would be (-39.3 +47.5)/200 = (+8.2/200) = +4.1% OB mix
For a more general rule you have to introduce also the relative % of each component.
In the case of a 3 component system:
OBA = oxygen balance % of compound A
OBB = oxygen balance % of compound B
OBC = oxygen balance % of compound C
wa = weight of compound A into x unit (for example g)
wb = weight of compound B into x unit (for example g)
wc = weight of compound C into x unit (for example g)
if:
1°) wm = weight of the sum of compounds A,B and C into the mix (also into x unit (for example g)).
2°) OBM = oxygen balance % of the mix A+B+C
then:
OBM = OBA*wa/wm + OBB*wb/wm + OBC*wc/wm
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Thraxx  | Hi,I would like to ask you,what you mean about the idea-
The Earl Warwicks Silly Putty, like plastificator.
Silly Putty invented 1943,composition of 65% dimethyl siloxane (hydroxy-terminated polymers with boric acid), 17% silica (crystalline
quartz), 9% Thixatrol ST (castor oil derivative), 4% polydimethylsiloxane, 1% decamethyl cyclopentasiloxane, 1% glycerine, and 1% titanium dioxide.
The idea to use silly putty like plastificator was publlished by amateur author Exxtec(Pyroforum),which described how to make a detonable
composition Silly Putty(28%)+Petn-RDX with spec.grav. 1,42 g/cm3.
One kind of this putty is full of Aluminium powder.And I think,that this kind of putty filled with Petn will under pressure of detonation
react between SiO2 + Al==Al2O3 + Si.
Can you tell me please,1)if such composition could be good? 2) if there will be DINGU on the place of Petn/Rdx,then could this a bit
acid putty be a stabilising factor for the Dingu?
Thank you Thraxx
[Edited on 15-10-2016 by Thraxx]
[Edited on 15-10-2016 by Thraxx] |
Like into many cases into the field of explosives, it is an unanswerable question without experimenting.
If I were you I would take contact with Exxtec from Pyroforum since he seems to have a practical knowledge of the platicizer in question.
I would avoid introducing much exogen compounds into an explosive mix ...simply remember that each added compound introduces a risk of side
chemical(s) reaction(s), is a source of complexity into the understanding of the detonation proces and may thus be a cause of storage unstability.
The role of the boric acid is probably the same as into polyhydric alcohol (like polyvinyl alcohol)/boric acid mix (do it self slime) --> to
increase coherence by -OH...HO- linkages...boric acid is boron trihydroxyde (B(OH)3 like Al(OH)3 but written H3BO3 to emphasize the fact it is an
acid).
If the Al react into the detonation zone (what is unlikely unless very finely dispersed (nanosized and coated or specialy processed to decrease
protective oxyd layer)) or if it reacts just behind or even into a late process maybe it will take a little of the oxygen from Si but remember that
the SiO2 will not be into a gaseous state...what strongly reduces the chances of reaction (a kind of thermite...what is usually small speed reaction
vs a detonation proces).
Dingu is less of an hydrolytic trouble than Tingu but acidity onto a microscopic molecular level might be a trouble or not...only experimentation can
tell (storage tests, accelerated decay/ageing into hot storage rooms, Differential Scanning Curve, Thermogravimetry)
[Edited on 16-11-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Tin man
Harmless

Posts: 34
Registered: 12-9-2016
Member Is Offline
Mood: Gruntled
|
|
Could 2,4,6-trinitrobenzoic acid be made be made by reacting TNT with a warm solution of sodium permanganate and sodium hydroxide. I'm hoping the TNT
would be Oxidized and dissolve as sodium 2,4,6-trinitrobenzoate. If so, the remaining Permanganate could be destroyed by dropwise addition of hydrogen
peroxide. Then the solution would be filtered to remove manganese dioxide, and the trinitrobenzoic acid could be precipitated using HCl, dissolved in
hot water, and reacted with your favorite heavy metal salt.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Tin man  | Could 2,4,6-trinitrobenzoic acid be made be made by reacting TNT with a warm solution of sodium permanganate and sodium hydroxide. I'm hoping the TNT
would be Oxidized and dissolve as sodium 2,4,6-trinitrobenzoate. If so, the remaining Permanganate could be destroyed by dropwise addition of hydrogen
peroxide. Then the solution would be filtered to remove manganese dioxide, and the trinitrobenzoic acid could be precipitated using HCl, dissolved in
hot water, and reacted with your favorite heavy metal salt.
|
You will have to try in tiny to get an answer.
Sodium or potassium permanganate are indeed oxydants for organic reactions into acidic media and into basic media aswel.
The usual procedure for TNToluen oxydation into TNBenzoic acid goes via acid/permanganate (avoid at all cost HCl what is
uncompatible with MnO4(-)...generates a lot of Cl2 gas (toxic suffocating war gas)).
This is part of the process of conversion of TNT into the more powerful TNBenzene...the last step is the decarboxylation by boiling water of the
TNBenzoic acid into TNBenzene...
HO2C-C6H2(NO2)3 --100°C--> C6H3(NO2)3 + CO2(g)
Since it is an extra step, it increases the cost of TNB vs TNT for military use; what explains TNT is favoured.
The decarboxylation occurs like in many cases into organic chemistry because of the Electron-withdrawing effect in alfa position vs the carboxylic
group (here the TNPhenyl moiety is EW)...same applies for the following groups -CO-, CCl3-, O2N-CH2-, HO2C-CH2-, ... what lead to easy thermal or even
spontaneous decarboxylation...the effect is enhanced by multiplication of the EWG groups into alfa, beta or further depending onto molecular structure
and electron transfer ability (molecular orbital).
Since the decarboxylation occurs quite easily under heating...I'm not sure you should heat up the TNBenzoic acid (or the TNBenzoate) or even dissolve
it into warm water...part of it will decompose and maybe the process is even easier for the salt in basic media?
Also don't heat up dry the basic media with TNT...a heat sensitive deep yellow sodium salt may form via aci-nitronate...the hydrogens atoms from the
methyl group are somewhat acidic (read labile) because of the EW ability of the TNPhenyl;
CH3-C=C-NO2 + NaOH <--==> CH2=C-C=N(O)-ONa + H2O (rest of the aromatic ring left aside for convenience purpose)
And this is evidenced by a lot of special reactions of TNT involving the CH3- and usually occuring in basic media like:
-halogenation (chloromethyl TNB, dichloromethyl TNB and trichloromethyl TNB),
-oxydation (TNBenzaldehyde, TNBenzoic acid),
-addition of aldehydes like formaldehyde (2-TNphenyl ethanol, TNStyrene, 2-TNphenyl-1,3-propandiol)
TNBenzene also is sensitized by strong bases and yellowing but the reaction is more obscure since there is no labile H so mechanism follows another
pathway.
[Edited on 16-11-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ill behaviour
Harmless

Posts: 8
Registered: 18-5-2016
Member Is Offline
Mood: No Mood
|
|
Thank you for answer, I am still at a very basic stage, so i just read away and try and learn.
Anyhow my latest question has to do with amine sensitized nitromethane. I read about people using epoxy as a source of amine. Can anyone fill me in on
what the finished product should look like? My NM went a clear yellow tint but when i gave it a good stir it went cloudy, any idea whats going on?
Also my epoxy seems to have multiple amines is this a safety issue.
Material Safety Data Sheet
H206 West System Slow Hardener
Version 1.1
Page 1 of 7
Section 1:
Identification of the Material and the Supplier
Trade Name
H206 West System Slow Hardener
Product Code
H206
Recommended Use
Used in conjunction with epoxy resin for adhesive and
composite application.
Company
ATL Composites
Address
12-14 Production Ave Ernest 4214
Telephone
+61 7 5563 1222 (Monday-Friday 8:30am-5:00pm)
Emergency Telephone
Number
+61 7 5563 1222 (Monday-Friday 8:30am-5:00pm)
Revision Date
22
n
d
May 2014
Section 2:
Hazards Identification
Hazard Classification:
HAZARDO
US SUBSTANCE : DANGEROUS GOODS
Risk Phrases
R34 Causes burns
R41
Risk of serious damage to eyes
R21/22
Harmful in contact with the skin and if swallowed
R42/43
May cause sensitization by inhalation and skin contact
R50/53
Very toxic to aquatic organisms, may cause long-term adverse
effects in the aquatic environment
Safety Phrases
S26
In case of contact with eyes, rinse immediately with plenty of water and seek
medical advice.
S28
After contact with skin, wash immediately with plenty of soap-suds.
S36/37/39
Wear suitable protective clothing, gloves and eye/face protection.
Section 3:
Composition / Information on Ingredients
Chemical Name
CAS No.
Weight %
Polyoxypropylenediamine
9046-10-0
30 - 60
Polymer of epichlorohydrin,
bisphenol-A, and DETA
31326-29-1
30 - 60
Tetraethylenepentamine
112-57-2
10 - <30
Reaction Products of TETA
and propylene oxide
26950-63-0 <10
Triethylenetetramine 112-24-3
<10
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
As far as I'm aware even the "most" sensitive PLX type composition of Nitromethane and and other Amines is around the sensitivity of C4 or RDX
(requiring strong initiation via a blasting cap or even booster) but I can't discount the possibility of side reactions with some of the other shit in
there like BPA or the other things. I'd be very surprised if it's become significantly sensitive to initiation but it is probably contaminated with
something, no clue what though.
Sorry if that's not too helpful.
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
ill behaviour
Harmless

Posts: 8
Registered: 18-5-2016
Member Is Offline
Mood: No Mood
|
|
Both products are cap sensitive but testing was done at night and it was a rush job so no useful information was obtained accept to say I was
completely amazed . I put 5 grams of mixed Amines in a jar and added 95 grams of 99% NM. The amine formed a layer on the top. After 24hours the NM
went yellow. I did the same thing in another jar but stirred the mix in a attempt to get the amine to completely dissolve in the NM. It got cloudy in
a major way. Also the amine/NM will not gel in smokeless powder like regular NM which is annoying so next thing is to try and gel it with Cabosil as
my microspheres just float in the liquid and my girlfriend is getting the shits with me trashing all her kitchen implements. I cant believe she has
memorized every item and questions me about the whereabouts of her utensils.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Get your own implements. Not hers.
If you use someone else's kitchen implements, much less the kitchen itself for your chemistry, you are asking to die. Not nescessarily from accidental
poisoning, fires caused by stove pilot lights, etc., but by the hand of a righteously pissed off person in charge of that kitchen...
"Honey, why is there a big yellow stain on the white counter top that won't clean up?" (Picric acid, dear.)
"Dear, what are all these black specs that show up whenever I try to use the blender?" (Honey, don't worry, it's just black powder.)
SMACK!
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Can anyone provide a description of the liquid explosives used in Russian PFM-1 type mines? All I find on searching are repeated references to " VS6-D
or VS-60D".
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
GarageExperiments
Harmless

Posts: 1
Registered: 6-12-2016
Member Is Offline
Mood: No Mood
|
|
PETN PBX
Hey, I'm completely new to the forum so hopefully this post isn't a step out of line or something that's already been over-discussed. I recently
synthesized a little batch of PETN and I would like to try to plasticize it. I'm thinking of using polybutene from Bird-B-Gone and some motor oil.
Will this combination work well or is it beneficial to add a phthalate or something similar as well? The professional PBX's usually contain something
like this. What general percentage range of plasticizer is acceptable for PETN? Also, what solvents are best? Acetone is out of the question
obviously, I was thinking ether/naptha, are these viable options?
[Edited on 7-12-2016 by GarageExperiments]
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Look up the ETN plasticizing thread
|
|
|
| Pages:
1
..
46
47
48
49
50
..
81 |