| Pages:
1
..
61
62
63
64
65
..
104 |
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
Thanks.
Im not usuing glassware, im using a steel pot.
Recrystalizatiom after the vinegar organic stuff burned didnt yield a pure product (I messed up quite a bit, it was my first recrystalization so
probably it could be done better)
I'll try roughly calculating the amount of liquid in the pot using the liquid level, but ill probably get a very bad yield.
[Edited on 12-9-2016 by ficolas]
|
|
|
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
I tried to crystalyce copper acetate into a big crystal dark crystal, like the ones seen here

(Image taken from SM btw)
But I can only get shitty small blue crystals.
I did get some dark ones starting to form in a copper wire, but a lot of blue ugly ones formed way faster.
What do I need to do to to get those beautiful crystals?
I thought about moving the evaporation thingie to a colder place, but that means it needs to be inside my house, something I dont think I can do. I
also thought about hanging a copper wire, since I saw dark ones starting on the copper wire, but since its something that takes so long, asking saves
me a lot of time 
|
|
|
zwt
Hazard to Self
 
Posts: 84
Registered: 1-8-2016
Member Is Offline
Mood: No Mood
|
|
Question
What tests can be done to determine the level of nitrate contamination in ammonium perchlorate made from ammonium nitrate and sodium perchlorate? I'd
imagine I could react away the ammonium with an excess of sodium hydroxide, and then reduce the nitrate to ammonia with aluminum, and then do
a Kjeldahl titration of the ammonia, but I'm looking for something that doesn't require destroying large samples of the ammonium perchlorate. Is there
any way to titrate for nitrate directly? This quote from the Wikipedia page "Nitrate test" gives cause for concern: | Quote: | | The nitrate anion is an oxidizer, and many tests for the nitrate anion are based on this property. Unfortunately, other oxidants present in the
analyte may interfere and give erroneous results. |
[Edited on 23-9-2016 by zwt]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by ficolas  | I tried to crystalyce copper acetate into a big crystal dark crystal, like the ones seen here
(Image taken from SM btw)
But I can only get shitty small blue crystals.
I did get some dark ones starting to form in a copper wire, but a lot of blue ugly ones formed way faster.
What do I need to do to to get those beautiful crystals?
I thought about moving the evaporation thingie to a colder place, but that means it needs to be inside my house, something I dont think I can do. I
also thought about hanging a copper wire, since I saw dark ones starting on the copper wire, but since its something that takes so long, asking saves
me a lot of time  |
Make sure you've got excess acetic acid in solution. Don't leave the copper wire in there, as you'll get a slow reaction with atmospheric oxygen to
give basic copper(II) acetate. I haven't had much luck with large beautiful crystals, but I tried leaving the copper wire in (actually, I was
suspending the seed crystal from a copper wire, since I could tie it more easily than fishing line) and it failed, failed failed for that reason.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Gemlingur
Harmless

Posts: 9
Registered: 7-9-2016
Member Is Offline
Mood: No Mood
|
|
Aqueous formalin
What would I expect from distilling a 37% formaldehyde solution? Is it an azeotrope?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
What's stopping you googling it?
|
|
|
Texium
|
Threads Merged
26-9-2016 at 08:34 |
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
Is this CuO or Cu2O??

The image doesn't show how black it is with lots of reflective "glitter like" speck in it. It is light and feels more like dry dirt.
I'm trying to figure out what I have here. It is the result of putting enameled copper wire (magnet wire) into hot (boiling at some points) molten
NaOH. It leaned up the wire to a beautiful shine but there seemed to be some black specks in the lye and on the copper as I shook it off as I removed
it from the pot.
I then soaked the copper w/ lye/black coating in hot water and boiled till clean. I added the water to the solidified lye until it was dissolved and
filterable. The remainder is what is left from all the windings I "cleaned". There is about 1.25 cup and it weighs about 83-85g and it is fluff y
like dirt. The thing is that I never had dirt near the lye or copper or solution pre-filter.
It has a dirt "feel" and a couple grains tasted like dirt. IT doesn't dissolve in water.
Any idea what this could be? Being so light how could it be copper (or is it some sodium compound)?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That might contain copper(II) oxide, but it doesn't look like it's even mostly copper(II) oxide.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It's probably from the enamel.
|
|
|
Adipocerex
Harmless

Posts: 8
Registered: 22-7-2016
Member Is Offline
Mood: No Mood
|
|
Need help identifying this secondary alcohol!
Can you guys help me identifying this orgo alcohol? Is it 2,3 dimethylpentanol? Or 2,dimethylhydroxypentanol.
https://scontent-arn2-1.xx.fbcdn.net/v/t34.0-12/14509282_102...
Thanks in advance
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
2-methyl-1,3-butanediol
Edit: to explain how to name it:
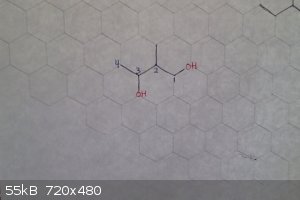
-Find the longest carbon chain that both hydroxyl groups are attached to, in this case, 4
-Number the carbons so that the substituents have the lowest numbers possible, in this case 1,2,3 (if you started at the other side it would be 2,3,4)
-Because there are two hydroxyl groups, it is classified as a diol
[Edited on 9-29-2016 by zts16]
|
|
|
Texium
|
Threads Merged
29-9-2016 at 05:53 |
Ba(ClO3)2
Harmless

Posts: 33
Registered: 30-4-2016
Member Is Offline
Mood: No Mood
|
|
Diethyl carbonate via sodium ethyl sulphate?
Would it be feasible to produce diethyl carbonate by dry distillation of sodium ethyl sulphate with sodium carbonate?
2 NaC2H5SO4 + Na2CO3 ==> (C2H5)2CO3 + 2 Na2SO4
The diethyl carbonate would be distilled off as it was formed leaving the sodium sulphate in the distilling flask.
If someone could confirm that this actually works, that would be great . .
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Possibly, but I wouldn't be surprised if you ended up with mostly ethylene gas (or diethyl ether).
|
|
|
Ba(ClO3)2
Harmless

Posts: 33
Registered: 30-4-2016
Member Is Offline
Mood: No Mood
|
|
ok, thanks anyway. we've got a very small amount of sodium ethyl sulphate which we want to use for something and we've always wanted to make some
diethyl carbonate (not sure why, its just interesting stuff).
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Does anyone have a good example of a simple and relatively safe runaway reaction?
I am putting together a series of demonstrations on reaction rates for my students. One of the concepts that we have looked at is the general rule
that reactions are often quick at first but then slow down as the reaction nears completion. But I want to show the students that there are
counterexamples.
I don't want to get into any complicated theories on autocatalysis but I would like to show a reaction that demonstrates a thermal runaway. The best
example that I can think of is solid state potassium permanganate and oxalic acid, but I would rather leave that one since I am using the solution
version of that reaction for a different demo.
My preference would be something in a liquid phase that is latent for a while and then takes off after a minute or so as the temperature rises. Any
suggestions?
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I think a good alternative might be potassium permanganate and glycerin - if I recall, that one starts reacting slowly and then speeds up to
conflagration in short order.
Another off the top of my head might be the 'carbon snake' demo, but this is tricky to do properly.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I am still after answers to my question above. But in the meantime I have another one.
Has anyone had any experience in working with hebel? It is a kind of aerated cement product used in construction. It can (apparently) be easily cut and carved with hand tools. Specifically,
I want to know how it will withstand high temperatures. I was considering constructing a kiln for small crucibles from a couple of hebel bricks:
Probably using a propane torch or maybe a small charcoal fire.
I don't think it is designed as a firebrick but it is used as a firewall in architectural applications. So it can withstand at least some heat. I am
attracted to it because it is cheap and readily available where I am and because I can quickly and easily carve it into the shapes I need. I envisage
something made from 2 or 3 bricks that holds a 30mm crucible and can vent a nice hot flame around the crucible.
I know that a bit of experimentation will give me my answer but was fishing for some experience first.
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
I had the exact same idea J and picked up some hebel bricks a couple of weeks ago, it is extremely easy to work (soft and porous like honey comb) I
initially tried to use it as the crucible when making the calcium carbide vie electric arc, it seemed to hold up under the temperature for the short
time that I tried, my issue was when the product became molten it disappeared into the porous material.
I don't have much faith in it holding up under extreme temperature, or many heat cycles but maybe a soak in sodium/potassium silicate could improve
its durability, something I’m yet to test, adding aluminium oxide powder to the mix might also help
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
j_sum1
although not entirely safe,
the reaction between cold conc. HNO3 and metals such as Cu and Ag
warns students of a common potential danger
it takes quite a while to start ... but when it does 
this video shows the dangers of both runaway and suckback https://www.youtube.com/watch?v=pJSQq494oV4
use copper that is not too finely divided, e.g. a copper coin.
I also like glycerine + KMnO4
[Edited on 7-10-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Any interesting Beryllium chemistry ?
I have some (yes I know it can be toxic) elemental berylium,
is there any interesting hobby-level beryllium chemistry that i can try ?
I have not found anything yet.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm under the impression that, other than its toxicity, beryllium chemistry is less interesting than aluminum.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
from what I've read so far Be and Al are chemically very similar,
even as part of catalytic compounds.
I'll just store it for now,
thanks.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
So much so that some of my really old books say that the best way to tell them apart is by tasting the compounds. Shudder.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
Excess toluene for benzyl chloride synth
I'm planning to make a small amount of benzyl chloride sometime soon. The chlorination of toluene in the presence of UV light is definitely the
easiest way for me. I plan to follow this procedure I found https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/in...
I've heard a fair excess of toluene is needed, otherwise significant amounts of benzal chloride and benzotrichloride are also produced. In the
procedure I linked to, a massive excess of toluene is used. Although the excess toluene is easily recycled, I would still prefer to use a minimum
amount of toluene.
what I'm asking is, roughly what is the minimum % excess of toluene I could use without any significant amount of other products of higher
chlorination forming?
Any advice would be much appreciated  . .
[Edited on 17-10-2016 by gluon47]
reality is an illusion 
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Latent heat of fusion experiment
I wish to have my students do an experiment that demonstrates latent heat of fusion. What I have in mind is to heat a solid to melting and then
monitor the temperature as it cools and solidifies. What should be observed is a flat spot on the temperature-time graph that corresponds to the
melting point. At this point the heat being released from the system comes from the phase change rather than a change in temperature.
I have done this before with naphthalene but as I recall the results were not amazingly clear. I am asking for suggestions for a material to use.
The ideal chemical would be:
something we have in stock
something that melts at a reasonable temperature -- 60°C would be ideal
something with a reasonably high latent heat of fusion
something that conducts heat well
I have considered water/ice but (a) boring and (b) I'd prefer a higher temperature
Gallium would be brilliant if we had buckets of the stuff
Sodium -- nice but ... nah. (Or should I say, "Na".) liquid sodium is not worth the risk assessment paperwork.
Tert butanol might be possible
Any other suggestions?
|
|
|
| Pages:
1
..
61
62
63
64
65
..
104 |