| Pages:
1
..
6
7
8
9
10
..
18 |
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Would melting sodium hydroxide in aluminium container cause that much problem? Certainly yes if the temperatures were much higher, but at about 300
degrees? Even if reactions were to occur, it would be the fusing of the surface of aluminium in contact with the molten sodium to form sodium
aluminate which would form sort of a protective layer, since it melts at extremely high temperatures.
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
tumadre
Hazard to Others
  
Posts: 172
Registered: 10-5-2005
Member Is Offline
Mood: No Mood
|
|
please read my previous post before answering this question.
Will propane react with the released CL2 in a non explosive manner as methane does? I am comsidering using propane as the shealding gas instead of co2
because three pounds of cl2 every five hours is too much, but 6-10 pounds of HCL and clorofoam is more easly disposed of/ or dissapated into the ...
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Of course you wouldn't get chloroform, you'd get CCl4 and other chlorinated but that is a minor point, the reaction of Cl2 with butane is
rapid at 30C in the presence of light and the reaction of methane with Cl2 is explosive (if stoichiometric) in the presence of light, but it's
hard to initate without light (or so I've been lead to belive) however.... it all depends on the tempreatures involved (which in your case are
~500 - 600C) so I'm at a loss for the specifics but I would say there is at least a noticable danger factor involved.
Why the need for a shielding gas? The Cl2 producing chamber is separated and even if it does react with some of the NaCl it will just electrolyze
again. H2 makes a good shielding gas for Na however it would explode from contact with Cl2.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I don't think activation energy is a problem, flames are awfully hot.
Does CCl4 decompose with heating? Is HCl preferred?
Tim
[Edited on 8-6-2005 by 12AX7]
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Carbon tet is fairly thermally stable, hence its old use in fire extinguishers but there is bound to be some decomposition at these temperatures.
Attached is THE ELECTROLYTIC PREPARATION OF THE AMALGAMS OF THE ALKALI AND ALKALI-EARTH METALS.
G. McP. Smith, H. C. Bennett;
J. Am. Chem. Soc.; 1909; 31(7); 799-806.
Kind of 'Cold Electrochemical Sodium' really but it is an interesting read and there has been somewhat significant intrest in the
preparation of sodium amalgams.
[Edited on 6/8/2005 by BromicAcid]
Attachment: alkalimetalamalgams.pdf (566kB)
This file has been downloaded 1497 times
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
While browsing through one of the volumes of <i>A Treatise on Chemistry</i> (thanks, S.C. Wack!) I noticed under an entry on potassium
that one suggested method for its electrochemical preparation is the electrolysis of fused KCN, by melting it completely, inserting electrodes,
waiting for the surface to form a crust of solid KCN as it cools, and then passing current. KCN has a lower melting point than the chloride, and has
the advantage that (unlike the hydroxide or chloride) the anode should not suffer attack. Might it work with NaCN as well?
But that actually wasn't the recommended method. The recommended method was to use a mixture of KCl and CaCl2 mixture with control of the heating
flame so that the area around the anode remained freely molten to expel chlorine, and the area around the cathode did not. After a period of current
passage, the mass of salts would be cooled and broken under liquid hydrocarbon to reveal potassium metal substantially free of calcium. It might be
possible to do the same with sodium compounds instead of potassium, and it would certainly be easier to prepare small amounts of sodium if no inert
shielding gas needs to be used.
PGP Key and corresponding e-mail address
|
|
|
Oxydro
Hazard to Others
  
Posts: 152
Registered: 24-5-2004
Location: NS, Canada
Member Is Offline
Mood: distracted
|
|
I was just browsing the eutectic-finder at http://ras.material.tohoku.ac.jp/~molten/molten_eut_query1.p... and I was wondering, what exactly is the problem with the GaCl3-NaCl mix? MP of
75-25 mixture is 55C -- I would assume that at that point gallium contamination would be a problem, but say at 50/50? what would happen?
"Our interest's on the dangerous side of things" -- Browning
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Gallium has the lower reduction potential, also any sodium formed would react with it.
Tim
|
|
|
Oxydro
Hazard to Others
  
Posts: 152
Registered: 24-5-2004
Location: NS, Canada
Member Is Offline
Mood: distracted
|
|
Pah. I posted without thinking.
Edit: or maybe with thinking backwards. I was leaving for work, so I don't really remember. Anyways obviously only such things as lithium,
potassium and calcium can be used, the only higher potentials according to the list I looked at.
[Edited on 23-6-2005 by Oxydro]
"Our interest's on the dangerous side of things" -- Browning
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Calcium yes, lithium maybe (you would probably get lithium instead), potassium no (this is a classic ohshititshoulntdothat moment in the making). You
cannot electrolyse a molten salt mixture of potassium and sodium to eather metal seperatly. Ive posted the reasons before as well as why try this is
particulally dangerous to try.
[Edited on 23-6-2005 by Marvin]
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Ahh! I typed a long response and the computer ate it. To put it simply, I used patu's method with an ATX 12v power supply, red devil lye, and a
copper anode and cathode. I used a propane torch to melt the NaOH around the anode and cathode, and then kept only the center of the NaOH molten
during the reaction. This prevents ALL corrosion from the outside container and prevents the sodium from overheating and forming that "grey
stuff". If the sodium hydroxide gets too hot, it will just melt more NaOH.
The copper anodes and cathodes held up much better than iron, and although the molten NaOH was turned blue, blue is a beautiful color (much better
than a brownish rusty color), and it didn't seem to affect anything.
There was splattering and popping, mostly at the beginning, after the loop on the cathode was mostly submerged, the popping was almost gone. I got a
few miniscule splats of NaOH on my gloves and arms (and face shield) but only one really hurt. It took off a few mm of skin in as many seconds.
I really recommend this method to make a bit of sodium- it's easy, inexpensive, and really pretty safe. Sure, you can get a tiny bit of NaOH on
you, but the reaction is very slow and controllable and so there's no danger of any deflagrations or anything spewing flaming mineral oil onto
people's heads...
Here's a diagram.
Cyrus
[Edited on 30-7-2005 by Cyrus]
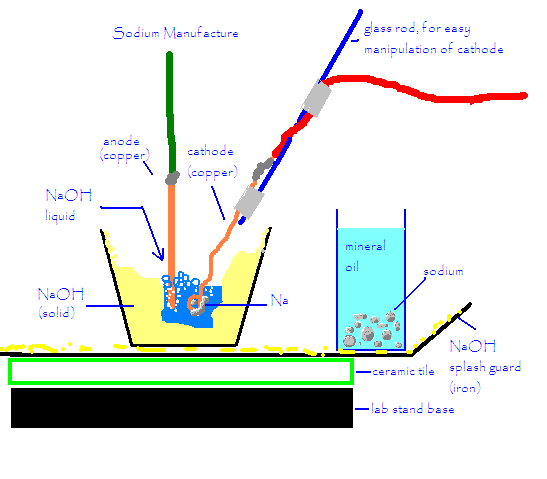
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
"Liver of sulfur"
Has anyone tried electrolyzing Na2S? Chatting with someone and mentioned its low MP...
Tim
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Low m.p. ?
Hardly, checking up its melting point via google reveals that it has a melting point of about 1000 degrees C, decomposing at that point.
The low "melting point" comes from the sodium sulfide monohydrate, having 50 degrees C. Beyond this, and the salt dehydrates.
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Ah. I'll have to slap my contact then 
Tim
|
|
|
tumadre
Hazard to Others
  
Posts: 172
Registered: 10-5-2005
Member Is Offline
Mood: No Mood
|
|
i am still here!
It has been a while, but this time I think I have the answer to this eight page question
sodium tetracloroaluminate.NaAlCl4.
It has a mp of just 151 C.
All I know about it is that it was first developted for liquid sodium-zinc and/or sodium-sulfur batterys, so why not just "charge the battery and
continualy draw off the sodium and add more NaCl or other sodium salts?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
That sounds like a hard substance to get. Anhydrous aluminum ions are tricky to make.
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Even if it is made it does not guarentee any properties that would be condutive to electrolysis, namely conductivity, anhydrous aluminum halides are
non-conductive, and although the sodium chloride makes itself into this compound, it may possess more covalent character then would allow for
electrolysis, its presence in these batteries being more as an overall solvent or what is being electrolyzed rather then something that can be
individually attacked with current to give sodium metal.
But it might be worth a shot, add NaCl to anhydrous AlCl3 probably right?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
If nothing else, if you assume it as AlCl3 in solution of molten NaCl, any Na formed will reduce the Al, forming Al metal, which I'm assuming is
your goal. If kinda inappropriate for this thead...
So yeah you can't make an NaX eutectic with anything but alkali and alkaline earth salts with a higher reduction potential, namely, Li, K, Rb,
Cs, Ca, Sr and Ba.
Hmm, most of those aren't that hard to come by. A quaternary or pentanary eutectic between Li, Na, K, Ca, Sr and Ba may melt as low as 200C.

Tim
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Mixtures of sodium and potassium salts bring the mp down spectaculally, but on electrolysis alloys are produced that are rather dangerous. You cannot
produce pure sodium or potassium metal from a mixture of the salts.
Sodium sulphide has a very high melting point, but it and sodium hydroxide have a eutectic thats a little lower than pure hydroxide, this has been
patented but I dont have the details.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I tried the standard electrolysis today: 5V, ~20A, 20mL liquid NaOH (red devil brand), all in a nickel crucible. The annode and cathode were both very
thick copper wire.
Electrolysis commenced with the standard period of violence and then progressed into a steady electrolysis with production of sodium and lots of tiny
NaOH droplets flying around (and landing on my hotplate, probably reducing its lifetime considerably).Temperature control was hard, but I think I
managed to keep it within acceptable limits for the most part.
I got that black solution early on, although this might have been a result of bismuth stuck to the crucible wall or temperature problems. It
didn't seem to give me any major problems.
The sodium formed at an appreciable rate, but here was my problem: I couldn't get it out very well. The eyedropper I had quickly got clogged with
NaOH and became unusable. My wire loop couldn't hold very much sodium because of its very low surface tension. Liquid NaOH kept solydifying on
it, so I had to keep breaking of this crust. As a result, I couldn't remove the forming sodium fast enough. I eventually switched to sticking
tweezers in the sodium and hoping to get as much sodium as I could where the NaOH solidified. I'll try to somehow recover my sodium tomorrow.
Is there something I'm missing here? I can't come up with a good way of removing the Na as fast as it forms.
I have and idea to fix this: confine the cathode (where the metal forms) in a glass tube with both ends open. The Na should form there and float at
the surface where it could collect without fear of shorting the circuit out. The only problem is how long would the glass tube last? I know that
liquid NaOH is notorious for destroying glass, but I don't know what kind of timeframe I'm looking at here (seconds, minutes, hours.) Also,
would the eroding glass interfere with the electrolysis?
P.S.
One more thing. When I was cleaning up my equiptment, I noticed that most things stayed basic after repeated washings. Does anyone anticipate any
problems with just leaving a small amount of NaOH there and letting the atmosphere do its work?
[Edited on 24-11-2005 by neutrino]
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
A glass tube could be used. It will not just dissolve quickly into your melt, likely your melt will solidify around the glass and not really attack
it, the problem being that if this happens the liquid within the tube will likely solidify as well, and if thats the case your sodium will rise up and
just go to the sides of the test tube, so you'd probably need a tube a little bit wider then you would expect.
[Edited on 11/24/2005 by BromicAcid]
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Why would the NaOH solidify? Heating from the electric current should easily keep it molten, right?
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
It was just my reasoning, that although glass is not really that conductive to heat, it might still be enough to wisk away some of the heat from the
melt and thereby cool it where it touched the glass causing it to freeze. However this may not be the case as the resistance from the heating of the
melt is right beneath the glass tube and the heat would rise and thereby melt the solid that would form there. So I guess you should just be wary
that something might happen along those lines.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I tried my idea a few days ago. In about a half hour of operation, the glass was eaten severely, some places had holes where I gently washed the
tubes.
My electrodes were both thick copper wire, both were submerged about 3cm into the melt. My yield was zero, I guess this has to do with those current
density issues mentioned higher up. How much 1mm diameter wire should be in my melt? The PSU was 20A @ 5V.
The NaOH turned blue after operation, indicating the annode was dissolving, although it doesn't look any thinner. The cathode was coated with a brown
substance, probably some copper that had plated on, albeit not well.
Temperature control was a problem here because everything kept freezing in my 40mL melt. I guess it doesn't help that it was below zero when I did
this. Next time I am doing it on a hot plate.
I did two successful runs with the loop method. I managed to collect about 1.5g of sodium of some unknown purity. It formed visible crystals when it
solidified under its oil. Does anyone know what sort of purity I might have here? I will upload a picture if anyone wants to see it.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Probably 99.9% or better, metals are pretty pure when they form a squarely crystalline regulus.
I've only seen it for example in 99.99% (1099 alloy) aluminum, which makes crystal formations up to 1/4" wide.
Tim
|
|
|
| Pages:
1
..
6
7
8
9
10
..
18 |