| Pages:
1
..
10
11
12
13
14
..
23 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by glymes  | Oh right... That rather changes things up!
So would it be [(C-NH2)-N3-NH]ClO4? The OB looks good. Indeed, would it be able to form nitroformate salts? I feel an investigation coming on....
|
Octonitrocubane, A nitrogen rich explosive, Eosin Y, Eosin Y again, Glymes or whatever new pseudo you will use...
The forum will be positively critical and it is not personnal, it is for your own good...that way you could progress and improve quality.
But I have the feeling you will do like you want, because you almost never took our advices seriously...this is proven by the chemical mistakes you
keep on posting the same way through your diverse ID's...
THINK Twice AND EVEN MORE before posting
Again you have lost a proton in your chemical story   
NH3 + HCl --> NH4Cl
N2H4 + HNO3 --> N2H5NO3
CH3-NH2 + HClO4 --> CH3-NH3ClO4
so
H2N-CN4H + HClO4 --> O4ClH3N-CN4H
PLEASE LEARN FROM YOUR REPETITIVE MISTAKES!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by glymes  | Right.
Perdifluoroaminohexamethyenetetramine. C6N16F24 --> 6CF4 + 8N2.
C6N4H12 + 12N-bromosuccinide --> C6N4Br12 + succinide
C6N4Br12 + 12HNF2 --> C6N16F24
Bromine is generally a good leaving group. For the sake, call it PDF HMTA, as I'd rather make it sound like a photocopier than a mouth orgy.
|
Octonitrocubane, A nitrogen rich explosive, Eosin Y, Eosin Y again, Glymes or whatever new pseudo you will use...
The forum will be positively critical and it is not personnal, it is for your own good...that way you could progress and improve quality.
But I have the feeling you will do like you want, because you almost never took our advices seriously...this is proven by the chemical mistakes you
keep on posting the same way through your diverse ID's...
THINK Twice AND EVEN MORE before posting
Again you made mistakes in your chemical story   
It is not because it works on paper that a reaction will work.
1°)Is there a trace of your putative per-bromination of HMTA (hexamethylenetetramine) via N-bromo succinimide somewhere into the chemical litterature
or on a website?
NO
=N-CH2-N= might be the representative linkage of HMTA and why would Br(+) attack the methylene and take H(+)?
2°) Is there a trace of your putative reaction of HNF2 with a geminal alkyl dibromide to make a geminal alkyl bis-difluoroamino compound into the
chemical litterature or on a website?
NO
3°) As explained by many at one of your previous ID's, the driving force of difluoroamino compounds is the formation of HF and this explains the
explosive power of those compounds, just like HCl is the driving force of -NCl2 (dichloroamines).
So no hydrogen and F linked to N what is slighly less electropositive than C (to make CF4) means no great reaction energy...so your compound might be
just as interesting as a teflon block...
PLEASE LEARN FROM YOUR REPETITIVE MISTAKES!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by glymes  | Hold up.
Where on earth are you fitting the dinitramide groups, as ANQ is NO2-(C=NH)-NH2? Would it just be [NO2(C=NH2)-NH2]- 2[NO2-N-NO2]+ or something like
that?
If that works, forming NO2CNHNH2(NO2-N-NO2]2 * H2O I see no reason why HO-NH2 should not be able to kick the H2O out and form NO2CNHNH2(NO2NNO2)2 *
HONH2
More neatly:
CH3N3O2 + NH3N3O4 --> CH3N6O6 + NH3
CH3N6O6 + H3NO --> C3H6N7O7 |
LEARN LEARN LEARN
ANQ is amino nitroguanidine, the formula has been posted many times and you are not even able to copy paste it!
Guanidine is (H2N-)2C=NH
Nitroguanidine is (H2N-)2C=N-NO2
Aminoguanidine is (H2N-)2C=N-NH2
Aminonitroguanidine is H2N-NH-C(-NH2)=N-NO2
(the double bond may change postion arround the C but then a H change also position)
What you wrote (NO2-(C=NH)-NH2) is C-nitro-formamidine (resulting form the putative addition of NH3 on nitryl cyanide).
Look at Klapote paper (provided by Microtek) on ANQ nitrate to learn the structure of ANQ dinitramide...you have again lost
a proton!
Quoted from you:
"If that works, forming NO2CNHNH2(NO2-N-NO2]2 * H2O I see no reason why HO-NH2 should not be able to kick the H2O out and form NO2CNHNH2(NO2NNO2)2
* HONH2"
If you see no reason:
-then maybe look at hydroxylamine pKa and pKb versus those of ANQ and follow the acido-basic rules (the stronger acid goes with the stronger base, the
weakest acid with the weakest base)
-then ask yourself why hydroxylamine dinitramide is not made yet
-then maybe change hobby
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
Actually, I stand corrected. That was a stupid post.
The following is how I would synthesise it right from the start:
CaCN2+NH4NO3-->[C(NH2)3]NO3
[C(NH2)3]NO3-dehydration with 98% H2SO4->NO2-N=C-(NH2)2 (probably nitroimine form as the resonance is stabilised compared to the nitroamine.)
NO2-N=C-(NH2)2+N2H4-->NO2-N-(NH2-)C-NH-NH2
And from there on:
NO2-N-(NH2-)C-NH-NH2+HN3-->NO2-N-(NH2-)C-NH-NH3-N3
3-amino 1-nitroguanidium azide would have around the following properties:
Nitrogen content by weight: 70%
Friction sensitivity: High
Shock sensitivity: High
Heat sensitivity: High
Can you see where this is going? I also expect that it would be both highly toxic and extremely explosive.
I also hypothesise that:
NO2-NH-(C-NH2)-N-NO2+N2H4-->NO2-NH-(C-NH-NH2)-N-NO2 (3-hydrazino 1,5-nitroguanidine.)
Therefore, by extension:
NO2-NH-(C-NH-NH2)-N-NO2+HN3-->NO2-NH-(C-NH-NH3)-N-NO2-N3
3-hydrazino 1,5-nitroguanidium azide. Which is a little bit silly.
And also (less nitrogen but better OB and density)
NO2-NH-(C-NH-NH2)-N-NO2+N-(NO2)2-->
[NO2-NH-(C-NH-NH2)-N-NO2]+ [NO2-N-NO2]-
3-hydrazino 1,5-nitroguanidium dinitramide.
I rest my case. I also don't think that any protons ran away!
[Edited on 22-5-2016 by glymes]
[Edited on 22-5-2016 by glymes]
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
These should make it clearer. If the following compounds do exist, they'd be spectacular explosives.
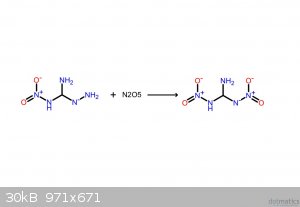 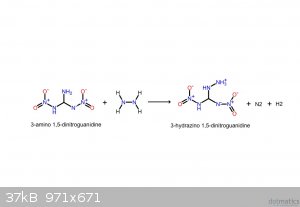
I believe that I screwed up the NH3: it's meant to be NH2, meaning that it takes NH from the hydrazine, yielding gaseous ammonia.
[Edited on 22-5-2016 by glymes]
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Need some help with ANQ nitrate. ANQ disolved instantly in large excess 67% HNO3 at 70ºC. Then I let it cool down but nothing crystallizes from the
solution, not even in the freezer. My nitric acid is very old, so probably is less than 67%. In the paper, HNO3 used was at 40%. IDK if it should be
more diluted or just wait.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
My final lot of theories.
1. NO2-N=C-(NH2)2 + N2H4 --> NO2-N=(C-NH2)-NH-NH2 + NH3
NO2-N-(C-NH2)-NH-NH2 can form salts. The azide (NO2-N-(C-NH2)-NH-NH2-N3 looks promising because of its high nitrogen content (69%.) It is also
predicted to be highly sensitive.
2. I also believe that NO2-N-(C-NH2)-NH-NH2 can be nitrated with dinitrogen pentoxide in chloroform, as suggested in Klapotke's paper:
NO2-N-(C-NH2)-NH-NH2 + N2O5 --> NO2-N-(C-NH2)-NH-NO2 + H2O + NO2 + N.
This would also be able to form salts, such as 3-amino 1,5-dinitroguanidium azide.
3. I also believe that NO2-N-(C-NH2)-NH-NO2 can be hydrazinolysed in the same way as (1) where the NH2 group is hydrazinolysed to NH-NH2 and free
ammonia gas.
NO2-N-(C-NH2)-NH-NO2 + N2H4 --> NO2-N-(C-NH-NH2)-NH-NO2 + NH3.
This could again form salts, such as 3-hydrazino 1,5-dinitroguanidium azide.
4. I also believe that NO2-N-(C-NH-NH2)-NH-NO2 may be able to be nitrated with N2O5 in chloroform in the same way as (2) in the following:
NO2-N-(C-NH-NH2)-NH-NO2 + N2O5 > NO2-N-(C-NH-NO2)-NH-NO2 + H2O + NO2+ N
This may also form salts such as 1,3,5-trinitroguanidium azide, which would be extremely explosive due to its high nitrogen content and high oxygen
balance.
5. If diaminonitroguanidine is synthesised and it can be protonated twice, it may form salts such as diaminonitroguanidium diazide,
NO2-N-C-(NH-NH2)-NH-NH2 2N3, or CH6N12O2. This would be extremely powerful also.
6. Some other salts may be formed from the hydrazinolysis of nitrourea:
NH2-(C=O)-NH-NO2 + N2H4 --> H2N-NH-(C=O)-NH-NO2.
If this forms salts, it may form 1-hydrazino 3-carboxyl 5-nitrourea nitrate, which would be a promising high explosive.
7. Potentially, 1-hydrazino 3-carboxyl 5-nitrourea may be able to be nitrated:
NH2-NH-(C=O)-NH-NO2 + NO2-BF4 --> NO2-NH-(C=O)-NH-NO2 + BF4
which could form 1,5-dinitro 3-carboxyl azide and other salts in the same vein.
I also theoreticise that nitration cannot go on for ever: it may not be possible to force a second nitro- group onto 1-hydrazino 3-carbonyl
5-nitrourea, even with nitronium tetrafluoroborate.
[Edited on 23-5-2016 by glymes]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by DubaiAmateurRocketry  |
It would be a decent oxidizer, however not good enough.
I personally do not like to introduce carbon atoms in the anion because they increase the overall average molecular weight of the exhaust in a
propellant.
If you take a look at the ANQ-dinitramide-monohydrate, it is very stable, has a VoD of nearly 9200, produces more gas than ANQ-Nitrate.
However if you put a nitroformate in there, the gas produced, L/kg will be significantly lower.
Edit:
Anyone have any idea if the ANQ-Dinitramide-monohydrate could be turned into... maybe ANQ-Dinitramide-monohydroxylamide ?
|
If the addition of C into a molecule induces heavier exhaust products and thus less good propelling abilities; it is not always bad for
detonic/explosive parameters....
See dinitrobenzene, trinitrobenzene and hexanitrobenzene...
HNB has a density of arround 2.0 and a VOD of 10000 m/s while it contains not a single H atom (what is mandatory for a good propellant) and it only
produces heavy gases like CO2 or CO and N2 or NxOy.
ANQ-Dinitramide-monohydrate can't be turned into ANQ-Dinitramide-monohydroxylamide because the hydroxylamine is more basic than the terminal H2N- of
the hydrazino moeity...
So HA will react with the dinitramidic acid and ANQ will form a zwiterionic compound (it contains both a basic and an acidic group that will
neutralize...reason why ANQ has to be neutralised in strong acid solutions to avoid precipitation of the zwiterionic species)
H2N-C(=N-NO2)-NH-NH3N(NO2)2 + HO-NH2 --> HO-NH3N(NO2)2 + O2N-N(-)-C(=NH)-NH-NH3(+)
The resulting HADN (hydroxylamine dinitramide) is probably too unstable to exist (never heard of it anywhere) and will then decompose into H2O, N2 and
NxOy.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by glymes  | These should make it clearer. If the following compounds do exist, they'd be spectacular explosives.
I believe that I screwed up the NH3: it's meant to be NH2, meaning that it takes NH from the hydrazine, yielding gaseous ammonia.
[Edited on 22-5-2016 by glymes] |
N2H4 will react as a base with the two acidic -NH-NO2 groups and will more likely not make the compound you want.
Hydrazine DiNitroGuanidate or Dihydrazine dinitroguanidate are for sure interesting to investigate.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Kratomiter: Remember that ANQ nitrate has a non-zero solubility in nitric acid. If you used "a large excess", you may simply not have enough product
to supersaturate the solution on cooling, in which case nothing will precipitate.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by DubaiAmateurRocketry  |
It would be a decent oxidizer, however not good enough.
I personally do not like to introduce carbon atoms in the anion because they increase the overall average molecular weight of the exhaust in a
propellant.
If you take a look at the ANQ-dinitramide-monohydrate, it is very stable, has a VoD of nearly 9200, produces more gas than ANQ-Nitrate.
However if you put a nitroformate in there, the gas produced, L/kg will be significantly lower.
Edit:
Anyone have any idea if the ANQ-Dinitramide-monohydrate could be turned into... maybe ANQ-Dinitramide-monohydroxylamide ?
|
If the addition of C into a molecule induces heavier exhaust products and thus less good propelling abilities; it is not always bad for
detonic/explosive parameters....
See dinitrobenzene, trinitrobenzene and hexanitrobenzene...
HNB has a density of arround 2.0 and a VOD of 10000 m/s while it contains not a single H atom (what is mandatory for a good propellant) and it only
produces heavy gases like CO2 or CO and N2 or NxOy.
ANQ-Dinitramide-monohydrate can't be turned into ANQ-Dinitramide-monohydroxylamide because the hydroxylamine is more basic than the terminal H2N- of
the hydrazino moeity...
So HA will react with the dinitramidic acid and ANQ will form a zwiterionic compound (it contains both a basic and an acidic group that will
neutralize...reason why ANQ has to be neutralised in strong acid solutions to avoid precipitation of the zwiterionic species)
H2N-C(=N-NO2)-NH-NH3N(NO2)2 + HO-NH2 --> HO-NH3N(NO2)2 + O2N-N(-)-C(=NH)-NH-NH3(+)
The resulting HADN (hydroxylamine dinitramide) is probably too unstable to exist (never heard of it anywhere) and will then decompose into H2O, N2 and
NxOy. |
Umm makes sense.
Hydroxylamine dinitramide has a stable zwiterionic form, I was very excited about it few years ago.
the zwiterionic form of hydroxylamine dinitramide exists in this manner:
[H3N+O-] [[NH3OH+]2]2+ [HONH2] [[N(NO2)2]2]2- Empirically N10H14O12.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
Ok.
Urea might hydrazinolyse:
NH2-(C=O)-NH2 + N2H4 --> NH2-NH-(C=O)-NH2
Which would then be able to form the nitrate:
NH2-NH-(C=O)-NH2 + NO2BF4 --> NH2-NH-(C=O)-NO2 + BF4
Which can form the azide upon addition of HN3:
NH2-NH-(C=O)-NO2 + HN3 --> [NH2-NH-(C=O)-NO2]+ N3- (I can't work out where the H should go?)
Empirically:
CH4N6O3, with a high nitrogen content.
Or we could hydrazinolyse the urea twice:
NH2-NH-(C=O)-NH2 + N2H4 --> NH2-NH-(C=O)-NH-NH2
And then nitrate this twice:
NH2-NH-(C=O)-NH-NH2 + 2NO2BF4 --> NO2-NH-(C=O)-NH-NO2 + 2BF4
And then this compound 3-carboxyl 1,5-dinitrourea could do the following:
NO2-NH-(C=O)-NH-NO2 + HN3 --> [NO2-NH-(C=O)-NH-NO2]+ N3- (still no bloody idea where the H goes) forming 1,5-dinitrourea azide (DNUA.)
Or, it could react with hydrazine again, forming hydrazine 1,5-dinitroureate (HDNU,) N2H5- [NO2-NH-(C=O)-NH-NO2]+.
DNUA = CH2N7O4
HDNU = CH7N6O4
Clearly, DNUA is the more promising explosive.
|
|
|
Cryolite
Harmless

Posts: 15
Registered: 20-4-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by glymes  | Ok.
Urea might hydrazinolyse:
NH2-(C=O)-NH2 + N2H4 --> NH2-NH-(C=O)-NH2
Which would then be able to form the nitrate:
NH2-NH-(C=O)-NH2 + NO2BF4 --> NH2-NH-(C=O)-NO2 + BF4
Which can form the azide upon addition of HN3:
NH2-NH-(C=O)-NO2 + HN3 --> [NH2-NH-(C=O)-NO2]+ N3- (I can't work out where the H should go?)
Empirically:
CH4N6O3, with a high nitrogen content.
Or we could hydrazinolyse the urea twice:
NH2-NH-(C=O)-NH2 + N2H4 --> NH2-NH-(C=O)-NH-NH2
And then nitrate this twice:
NH2-NH-(C=O)-NH-NH2 + 2NO2BF4 --> NO2-NH-(C=O)-NH-NO2 + 2BF4
And then this compound 3-carboxyl 1,5-dinitrourea could do the following:
NO2-NH-(C=O)-NH-NO2 + HN3 --> [NO2-NH-(C=O)-NH-NO2]+ N3- (still no bloody idea where the H goes) forming 1,5-dinitrourea azide (DNUA.)
Or, it could react with hydrazine again, forming hydrazine 1,5-dinitroureate (HDNU,) N2H5- [NO2-NH-(C=O)-NH-NO2]+.
DNUA = CH2N7O4
HDNU = CH7N6O4
Clearly, DNUA is the more promising explosive. |
BF4? What is BF4? I don't think a single one of your proposed "equations" is balanced.
Before you risk maiming or poisoning yourself with such dangerous reagents (NO2BF4!! HN3!!!), and before you attempt to propose novel explosive
compounds and lecture others on safety, I advise you to sit down and actually learn chemistry from the basics, especially if you want to be taken at
all seriously here.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
And finally the pinnacle of insanity. Triaminoguanidium diazide.
[NH2-NH-(C-NH-NH2)-NH-NH2]+ 2N3- or, empirically, CH9N12. 89% N.
I believe that this compound is like an angry genie. Tap on your round bottomed flask three times and the genie will appear with a loud report, a puff
of nitrogen and a shower of glass.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Your charges don't balance.
I'm not sure this sort of chemistry by scrabble rules discussion is beneficial to anyone.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
Yes it is. It is a discussion about theoretical energetics. And therefore, Scrabble rules can yield interesting results.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Drawing new energetics is certainly fun Glymes, but not exactly useful if the theoretical compounds aren't investigated a bit further.
First off: could the molecule even exist in reality? This is pretty basic, counting hydrogens and balancing charges. Looking up pKa if it's a salt
could be a good idea; your triaminoguanidinium diazide would not keep together for example.
Second: would it be worth the hassle to try to make this material? This means either a VoD > 9 km/s or an extremely insensitive energetic. So
calculate the density with your method of choice (I like a fragment addition method), predict the enthalpy of formation with a quantum chemistry
program like FireFly and finally calculate the detonation performance. This can be done with a simple formula like Kamlet-Jacobs.
Third: synthesis. Presenting something like polynitrogen ( -[NH-NH]-, VoD ~30 km/s) is useless unless there is at least an idea for a synthesis. These
should of course be based on the literature. It will likely take multiple trials to get a working, preferably high yield, route to the new compound.
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Looks like Nitrogen rich explosive is back.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Microtek  | | According to a paper by Castillo-Melendez and Golding, the yield of ANQ can be as high as 60% given the right reaction conditions (55 C, 15 minutes
reaction time). The paper is from 2004, so it's older than the one I posted by Klapötke, et al. This could indicate that it is quite difficult to
reach those numbers. I think this is supported by the fact that the yield peak is quite narrow. |
Thank you for that paper too!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by DubaiAmateurRocketry  | Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by DubaiAmateurRocketry  |
It would be a decent oxidizer, however not good enough.
I personally do not like to introduce carbon atoms in the anion because they increase the overall average molecular weight of the exhaust in a
propellant.
If you take a look at the ANQ-dinitramide-monohydrate, it is very stable, has a VoD of nearly 9200, produces more gas than ANQ-Nitrate.
However if you put a nitroformate in there, the gas produced, L/kg will be significantly lower.
Edit:
Anyone have any idea if the ANQ-Dinitramide-monohydrate could be turned into... maybe ANQ-Dinitramide-monohydroxylamide ?
|
If the addition of C into a molecule induces heavier exhaust products and thus less good propelling abilities; it is not always bad for
detonic/explosive parameters....
See dinitrobenzene, trinitrobenzene and hexanitrobenzene...
HNB has a density of arround 2.0 and a VOD of 10000 m/s while it contains not a single H atom (what is mandatory for a good propellant) and it only
produces heavy gases like CO2 or CO and N2 or NxOy.
ANQ-Dinitramide-monohydrate can't be turned into ANQ-Dinitramide-monohydroxylamide because the hydroxylamine is more basic than the terminal H2N- of
the hydrazino moeity...
So HA will react with the dinitramidic acid and ANQ will form a zwiterionic compound (it contains both a basic and an acidic group that will
neutralize...reason why ANQ has to be neutralised in strong acid solutions to avoid precipitation of the zwiterionic species)
H2N-C(=N-NO2)-NH-NH3N(NO2)2 + HO-NH2 --> HO-NH3N(NO2)2 + O2N-N(-)-C(=NH)-NH-NH3(+)
The resulting HADN (hydroxylamine dinitramide) is probably too unstable to exist (never heard of it anywhere) and will then decompose into H2O, N2 and
NxOy. |
Umm makes sense.
Hydroxylamine dinitramide has a stable zwiterionic form, I was very excited about it few years ago.
the zwiterionic form of hydroxylamine dinitramide exists in this manner:
[H3N+O-] [[NH3OH+]2]2+ [HONH2] [[N(NO2)2]2]2- Empirically N10H14O12.
|
Interesting DAR.
Do you have the physico-chemical detonic/propellant properties of that compound under hand? Or the paper about it?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by glymes  |
NO2-N-(NH2-)C-NH-NH2+HN3-->NO2-N-(NH2-)C-NH-NH3-N3
3-amino 1-nitroguanidium azide
I also hypothesise that:
NO2-NH-(C-NH2)-N-NO2+N2H4-->NO2-NH-(C-NH-NH2)-N-NO2 (3-hydrazino 1,5-nitroguanidine.)
Therefore, by extension:
NO2-NH-(C-NH-NH2)-N-NO2+HN3-->NO2-NH-(C-NH-NH3)-N-NO2-N3
3-hydrazino 1,5-nitroguanidium azide.
NO2-NH-(C-NH-NH2)-N-NO2+N-(NO2)2-->
[NO2-NH-(C-NH-NH2)-N-NO2]+ [NO2-N-NO2]-
3-hydrazino 1,5-nitroguanidium dinitramide.
I rest my case. I also don't think that any protons ran away! |
If ANQ needs > 40% HNO3 or HClO4 (what are very strong acids with pKa <-2) to be protonated and avoid zwiterionic autoprotonation...then the
poorly acidic HN3 (pKa = 4,6 what is at least 107 times order of magnitude less acidic than HNO3 and HClO4 (107= 10.000.000
times)) won't be able to join the molecule and freebase gas you to death!
You obviously can't read! I already explained that the dinitroguanidine will simply form a salt of hydrazine, a mono hydrazine or a dihydrazine salt,
no NH3 will escape to make your compound.
In your last formula you lost a proton again... hydrogen dinitramide is HN(NO2)2
Quote: Originally posted by glymes  |
NO2-N-(C-NH2)-NH-NH2 can form salts. The azide (NO2-N-(C-NH2)-NH-NH2-N3
2. I also believe that NO2-N-(C-NH2)-NH-NH2 can be nitrated with dinitrogen pentoxide in chloroform, as suggested in Klapotke's paper:
NO2-N-(C-NH2)-NH-NH2 + N2O5 --> NO2-N-(C-NH2)-NH-NO2 + H2O + NO2 + N.
This would also be able to form salts, such as 3-amino 1,5-dinitroguanidium azide.
3. I also believe that NO2-N-(C-NH2)-NH-NO2 can be hydrazinolysed in the same way as (1) where the NH2 group is hydrazinolysed to NH-NH2 and free
ammonia gas.
NO2-N-(C-NH2)-NH-NO2 + N2H4 --> NO2-N-(C-NH-NH2)-NH-NO2 + NH3.
This could again form salts, such as 3-hydrazino 1,5-dinitroguanidium azide.
4. I also believe that NO2-N-(C-NH-NH2)-NH-NO2 may be able to be nitrated with N2O5 in chloroform in the same way as (2) in the following:
NO2-N-(C-NH-NH2)-NH-NO2 + N2O5 > NO2-N-(C-NH-NO2)-NH-NO2 + H2O + NO2+ N
This may also form salts such as 1,3,5-trinitroguanidium azide, which would be extremely explosive due to its high nitrogen content and high oxygen
balance.
5. If diaminonitroguanidine is synthesised and it can be protonated twice, it may form salts such as diaminonitroguanidium diazide,
NO2-N-C-(NH-NH2)-NH-NH2 2N3, or CH6N12O2. This would be extremely powerful also.
6. Some other salts may be formed from the hydrazinolysis of nitrourea:
NH2-(C=O)-NH-NO2 + N2H4 --> H2N-NH-(C=O)-NH-NO2.
If this forms salts, it may form 1-hydrazino 3-carboxyl 5-nitrourea nitrate, which would be a promising high explosive.
7. Potentially, 1-hydrazino 3-carboxyl 5-nitrourea may be able to be nitrated:
NH2-NH-(C=O)-NH-NO2 + NO2-BF4 --> NO2-NH-(C=O)-NH-NO2 + BF4
which could form 1,5-dinitro 3-carboxyl azide and other salts in the same vein.
I also theoreticise that nitration cannot go on for ever: it may not be possible to force a second nitro- group onto 1-hydrazino 3-carbonyl
5-nitrourea, even with nitronium tetrafluoroborate.
|
All wrong again
1°)ANQ can only form salts with very strong acids. HN3 is way to weak .
2°)Why nitrate ANQ (made from NQ) to get DNQ what can be made directly from NQ (and that is what is written in Klapote's paper...but yes of course
you can't read)
A dinitro(amino)guanidine would be even more acidic than ANQ and so formation of an azide is even more elusive.
3°)DNQ won't hydrazinolyse, it will form a (di)hydrazinium salt.
Here also no HN3 salt possible!
4°) Trinitroguanidine TNQ is only theorical at this date, it will be super acidic vs DNQ and so forming a HN3 salt of it...you know the song now...
5°) If you have chance DANQ or DANG (diaminonitroguanidine) and that it is basic enough then maybe one HN3 will fix on it, certainly not 2.
Here also you forgot 2 protons NO2-N=C-(NH-NH3N3)-NH-NH3N3
6°) Hydrazinolysis of nitrourea?
Is nitrourea stable enough to hydrazinolyse?
NU and DNU are sensitive to water already...DNU forms NH4 and K salts in the cold with 50% loss in the process by hydrolysis...
Why would N2H4 favor NH3 elimination and not NH2-NO2 elimination?
7°)"1-hydrazino 3-carboxyl 5-nitrourea" There is something all wrong with the numbers of that formula.
Why start from 3-amino-1-nitrourea to make 1,3-dinitrourea while it can be done directly from urea?
Again Ochus Pochus NH2 disappear as dark matter...
BF4NO2 = BF3 + F-NO2 = BF4(-)NO2(+) = nitronium salt
Why would a NO2(+) replace a NH2(+) (very hard if not impossible to make)?
No HN3 will join in a salt with DNU...which is way too acidic!
8°)"I also theoreticise that nitration cannot go on for ever: it may not be possible to force a second nitro- group onto 1-hydrazino 3-carbonyl
5-nitrourea, even with nitronium tetrafluoroborate."
Wow, so your daydreaming have limits...glad to read that...and at least something you wrote that is right!
Quote: Originally posted by glymes  |
Which would then be able to form the nitrate:
NH2-NH-(C=O)-NH2 + NO2BF4 --> NH2-NH-(C=O)-NO2 + BF4
Which can form the azide upon addition of HN3:
NH2-NH-(C=O)-NO2 + HN3 --> [NH2-NH-(C=O)-NO2]+ N3- (I can't work out where the H should go?)
Empirically:
CH4N6O3, with a high nitrogen content.
Or we could hydrazinolyse the urea twice:
NH2-NH-(C=O)-NH2 + N2H4 --> NH2-NH-(C=O)-NH-NH2
And then nitrate this twice:
NH2-NH-(C=O)-NH-NH2 + 2NO2BF4 --> NO2-NH-(C=O)-NH-NO2 + 2BF4
And then this compound 3-carboxyl 1,5-dinitrourea could do the following:
NO2-NH-(C=O)-NH-NO2 + HN3 --> [NO2-NH-(C=O)-NH-NO2]+ N3- (still no bloody idea where the H goes) forming 1,5-dinitrourea azide (DNUA.)
|
Again you have lost some NH2 somewhere over the rainbow!
The molecule H2N-NH-CO-NO2 would be too unstable to exist and is not nitrourea or aminonitrourea what should be H2N-CO-NH-NO2 and H2N-NH-CO-NH-NO2.
Nitrate is from nitric acid -O-NO2 or ONO2(-) or NO3(-)...-NO2 is nitro. To make semicarbazine (aminourea) or carbazine (diaminourea) nitrate or
dinitrate is simply a matter of adding dilluted HNO3
ANU (aminonitrourea) and DNU would be too acidic to make a salt with HN3!
Quote: Originally posted by glymes  | And finally the pinnacle of insanity. Triaminoguanidium diazide.
[NH2-NH-(C-NH-NH2)-NH-NH2]+ 2N3- or, empirically, CH9N12. 89% N.
I believe that this compound is like an angry genie. Tap on your round bottomed flask three times and the genie will appear with a loud report, a puff
of nitrogen and a shower of glass. |
Maybe TAG (triaminoguanidine) will form a mono hydrazoate or a dihydrazoate.
H2N-N=C(-NH-NH3N3)-NH-NH2
and
H2N-N=C(-NH-NH3N3)2 depending on the pKb1, pKb2 and pKb3 of TAG.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by DubaiAmateurRocketry  | Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by DubaiAmateurRocketry  |
It would be a decent oxidizer, however not good enough.
I personally do not like to introduce carbon atoms in the anion because they increase the overall average molecular weight of the exhaust in a
propellant.
If you take a look at the ANQ-dinitramide-monohydrate, it is very stable, has a VoD of nearly 9200, produces more gas than ANQ-Nitrate.
However if you put a nitroformate in there, the gas produced, L/kg will be significantly lower.
Edit:
Anyone have any idea if the ANQ-Dinitramide-monohydrate could be turned into... maybe ANQ-Dinitramide-monohydroxylamide ?
|
If the addition of C into a molecule induces heavier exhaust products and thus less good propelling abilities; it is not always bad for
detonic/explosive parameters....
See dinitrobenzene, trinitrobenzene and hexanitrobenzene...
HNB has a density of arround 2.0 and a VOD of 10000 m/s while it contains not a single H atom (what is mandatory for a good propellant) and it only
produces heavy gases like CO2 or CO and N2 or NxOy.
ANQ-Dinitramide-monohydrate can't be turned into ANQ-Dinitramide-monohydroxylamide because the hydroxylamine is more basic than the terminal H2N- of
the hydrazino moeity...
So HA will react with the dinitramidic acid and ANQ will form a zwiterionic compound (it contains both a basic and an acidic group that will
neutralize...reason why ANQ has to be neutralised in strong acid solutions to avoid precipitation of the zwiterionic species)
H2N-C(=N-NO2)-NH-NH3N(NO2)2 + HO-NH2 --> HO-NH3N(NO2)2 + O2N-N(-)-C(=NH)-NH-NH3(+)
The resulting HADN (hydroxylamine dinitramide) is probably too unstable to exist (never heard of it anywhere) and will then decompose into H2O, N2 and
NxOy. |
Umm makes sense.
Hydroxylamine dinitramide has a stable zwiterionic form, I was very excited about it few years ago.
the zwiterionic form of hydroxylamine dinitramide exists in this manner:
[H3N+O-] [[NH3OH+]2]2+ [HONH2] [[N(NO2)2]2]2- Empirically N10H14O12.
|
Interesting DAR.
Do you have the physico-chemical detonic/propellant properties of that compound under hand? Or the paper about it? |
I forgot about the sensitivity, I would guess not too crazy since there are a good amount of strong hydrogen bonds. But again, both hydroxylammonium
and dinitramide tend to produce compounds toward the high sensitivity side. So I am not sure
Here is a paper for this particular compound:
http://link.springer.com/article/10.1023%2FA%3A1011362427996
I remember I read about it a while ago, I dont seem to own the paper anymore and forgot what I read. I just left my institute so I cannot use the
library internet to open it anymore so I'd appreciate it if anyone could share the open version.
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
After more than 12 hours in the freezer a little amount crystallized. It was my fault: large excess of nitric acid. Once filtrated, I recrystallized
it again from hot water to remove any nitric acid. Once filtrated, it dried quickly. It's like cotton (like nitroguanidine is) and very thin. IDK if
that's really ANQ nitrate, I'll do some test tomorrow (litle more than burning it).
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by kratomiter  | | After more than 12 hours in the freezer a little amount crystallized. It was my fault: large excess of nitric acid. Once filtrated, I recrystallized
it again from hot water to remove any nitric acid. Once filtrated, it dried quickly. It's like cotton (like nitroguanidine is) and very thin. IDK if
that's really ANQ nitrate, I'll do some test tomorrow (litle more than burning it). |
Theoretically, if you burned it, it would leave no ash/tar behind since its OB is perfect (I might be wrong though).
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
I diluted the solution with water to make it easy to crystallize, but this can make unprotonated ANQ to crystallize also. Heated by flame on aluminium
foil it releases lots of white fumes, leaving litle residue.
I don't have any equipment to analyze it, but could be an interesting propellant if it's actually ANQ nitrate.
|
|
|
| Pages:
1
..
10
11
12
13
14
..
23 |