| Pages:
1
..
36
37
38
39
40
..
81 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by woelen  | C3N3Cl3H3???
What is this? This cannot be an existing cyclic compound. We have C3N3Cl3 (cyanuric chloride) and C3N3Cl3O3 (TCCA) but not C3N3Cl3H3.
|
Because if you take the time to make a beautiful drawing; then take a minute to count each atom kind not to make mistakes into your équations...
Like I said many times to your other IDs:
Think twice and even more before posting aberrations.
Use the search engine!
Trinitroisocyanuric and trinitrocyanuric rings have been discussed many times.
[Edited on 17-5-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
theory
I just finished the special theory of relativity for all the deminers. E = O . H2.
And vice versa: O . H2 = E. Respectively VoD is about 3200m/s. Without any detonator. Only hot wire. Maybe will be good a only warm wire. Short and
simply. And mix worked always. Is it relatively bullshit, but is it contribution. https://deepblue.lib.umich.edu/bitstream/handle/2027.42/3730...
... ...LL ...LL
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
One quick question: I want to try the synthesis of guanidine perchlorate using ammonium perchlorate instead of perchloric acid.
NH2C(=NH)NH2 · ½H2CO3 + NH4ClO4 -> NH2C(=NH)NH2ClO4 + NH3 + CO2 + H2O
At high temperatures (>80ºC) in water solution NH3 and CO2 will leave the system, shifting the reaction to the right. Is this correct? Synthesis
of HClO4 is not very amateur-friendly and I want to avoid it.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Thanks for that H2/O2 paper Liptakov! It confirms that my BKW implementation (calibrated for solid/liquid explosives!) works exceptionally well for
gaseous mixtures too!
The graph is from that report above; the red dots are my calculated values.
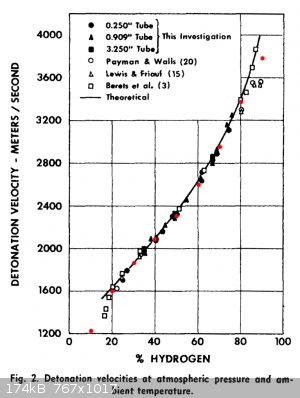
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by kratomiter  | One quick question: I want to try the synthesis of guanidine perchlorate using ammonium perchlorate instead of perchloric acid.
NH2C(=NH)NH2 · ½H2CO3 + NH4ClO4 -> NH2C(=NH)NH2ClO4 + NH3 + CO2 + H2O
At high temperatures (>80ºC) in water solution NH3 and CO2 will leave the system, shifting the reaction to the right. Is this correct? Synthesis
of HClO4 is not very amateur-friendly and I want to avoid it. |
Should work since guanidine is a stronger base than NH3 and HClO4 a stronger acid than H2CO3.
Then:
(H2N-)2C=NH.H2CO3 + NH4ClO4 <--==> (H2N-)2C=NH.HClO4 + NH4HCO3
NH4HCO3 -heat-> NH3 + CO2 + H2O
Maybe to avoid suffocating allow the fumes to pass over agitated saturated CuSO4 solution...it will catch the H2O, the CO2 and the NH3
CuSO4(aq) + 4 NH3 (g) --> Cu(NH3)4SO4(aq)
CuSO4 + H2CO3 --> CuCO3(s) + H2SO4
NH3 + H2SO4 --> NH4HSO4
NH4HSO4 + NH3 --> (NH4)2SO4
Alternatively you may use CaCl2
CaCl2 + NH3(g) --> CaCl2.x NH3
CaCl2 + H2CO3 --> CaCO3(s) + 2 HCl
HCl + NH3 --> NH4Cl
Do not allow the tube to go into the CuSO4 or CaCl2 solution or it will be sucked up because NH3 has a big affinity for H2O and for the complex -->
this would cause a depression and CuSO4 or CaCl2 solution would pass into the initial reactor.
[Edited on 18-5-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
H20
Well! The theory of water confirmed. Velocity of detonation is even bigger , than I'm waiting. Your counting system is working precise. Good picture
insert points, Dorni. Short question: How is highest VoD for H2+ 02? Graph is not ended upstairs. I'm estimate, that is it interesting question for
all. Thanks.. ....LL ....LL
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
What explosive are you talking about? Minemanit? Glycolex?
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Good question indeed Liptakov.
I ran these calculations for a gas mixture at 1.03 bar and 298 K.
| Code: |
Highest detonation velocity: 3725 m/s 90.7% H2, 9.3% O2
Highest detonation temperature: 3457 K 67.2% H2, 32.8% O2
Highest detonation pressure: 18.9 bar 69.1% H2, 30.9% O2 |
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
EM
Huuh.. Thanks for everybody. Highest press only 19 Bars? However if is full for example room, good pressure.
Next, glymes: Minemanit is EM, inventor MineMan. Glycolite (not glycolex) is EM, inventor Dr. Liptakov... ...looking on YT. ...looking on YT.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
Ah. Minemanit looks pretty good...
I'll U2U you one of my ideas.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by glymes  | True or false?
And by the same token trichloroacetic acid + nitric acid = trinitroacetic acid?
The above would be better because it would give a higher oxygen percentage, allowing for more explosive, more dense transition metal salts.
[Edited on 16-5-2016 by glymes] |
So because Glymes (alias octonitrocubane, A nitrogen rich explosive, Eosin Y, Eosin Y again) doesn't do his homeworks.
He wrote:
1)Cl2C-CO2H -HNO3-> (O2N-)2C-CO2H
2)dichloroacetic acid + nitric acid --> dinitroacetic acid
3)C2H2Cl2O2 + 2 HNO3 --> C2H2N2O4
4)C2H2N2O4 + 2KOH --> KC2H2N2O4
But this is all wrong!
1°) dichloroacetic acid is Cl2CH-CO2H (to respect tetravalence of C)
2°) HNO3 will more likely attack the CH from Cl2CH- and as explained earlier will form Cl2C(NO2)-CO2H; this carboxylic acid holding strong electron
withdrawing groups (EWG) will easily decarboxylate...
This is observed:
-in the lab making of nitromethane from chloroacetic acid and NaNO2 (the transcient nitroacetic acid is destroyed),
-in pyruvic acid that easily turns into ethanal + CO2,
-in malonic acid that easily turns into acetic acid + CO2
-in easy transformation of trinitrobenzoic acid into TNB + CO2
3°) If the equation 3 was true; then dinitroacetic acid would be C2H2N2O6 (by magic power Glymes made 2 oxygen disappear!)
4°) If equation 4 was true; then magically again you involve 2 KOH into acido-basic reactions but only get one attached; the second is gone as dark
matter...
C2H2N2O6 + 2KOH --> C2K2N2O6 + 2 H2O
Again writing equation is gross molecular formula increases the risk of mistakes. You should opt for semi-developped formulas.
In chemistry the matter is conserved so you must have the same number of atoms of each kind on both sides of the equation
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  |
And magically TADAAAHHHH...
the sodium disappeared from the equation...
Glymes aka the multiple ID poster is also an alchemist magician transmuting sodium to anti-matter...
How is nitromethane done from chloroacetic acid and NaNO2?
Search for the equation and transpose to mono-substitution and disubstitution...
What could be the side products since substitution is not 100% effective? 60%-30% depending on conditions...
The other product is hydrolysable into what?
[Edited on 17-5-2016 by PHILOU Zrealone] |
Because Glyme aka the multiple ID poster has not done his homework:
2NaNO2+2HCl+CH(Cl)2(COOH) --> CH(NO2)2(COOH)+2HCl
1°) Use arrows instead of =
2°) Why use HCl?
In chemical equations what is on both sides can be supressed so:
2NaNO2+CH(Cl)2(COOH) --> CH(NO2)2(COOH)+2 NaCl
3°) 2 Cl-CH2-CO2H + 2 NaNO2 --> O2N-CH2-CO2H + O=N-O-CH2-CO2H + 2 NaCl
O2N-CH2-CO2H --> CH3-NO2 + CO2(g)
So
2 Cl2CH-CO2H + 2 NaNO2 --> O2N-CHCl-CO2H + O=N-O-CHCl-CO2H + 2 NaCl
then
O2N-CHCl-CO2H --> CH2Cl-NO2 + CO2(g)
O=N-O-CHCl-CO2H + H2O <==> HONO + HOCHCl-CO2H
HOCHCl-CO2H <==> HCl + O=CH-CO2H
O=CH-CO2H -oxydant like HNO2-> HO2C-CO2H
CH2Cl-NO2 + HONO --> O=N-CHCl-NO2 + H2O
then
2 CH2Cl-NO2 + 2 NaNO2 --> CH2(-NO2)2 + O=N-O-CH2-NO2 + 2 NaCl
O=N-O-CH2-NO2 + H2O --> HONO + HO-CH2-NO2
HO-CH2-NO2 <==> HONO + CH2=O
CH2=O -oxydant like HNO2-> H2CO3 --> H2O + CO2
CH2(-NO2)2 + HONO --> O=N-CH(-NO2)2 + H2O <==> HO-N=C(-NO2)2 + H2O
HO-CH2-NO2 + HONO --> HO-C(=NOH)-NO2 + H2O --> CO2 + H2O + N2
HO-N=C(-NO2)2 -more likely-> CO2 + H2O + NxOy
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by Eosin Y again  | Some thoughts.
1) Acetic acid + tetranitromethane = trinitroacetic acid?
CH3COOH+C(NO2)4=C(NO2)3COOH+CH3NO2 (which would bubble out of solution.)
2) Ammonium chloride + trinitroacetic acid = dinitroaminoacetic acid?
NH4Cl+C(NO2)3COOH=C(NO2)2COONH3+HCl
These might be rubbish because I am just speculating. |
Right! Speculative full of mistakes irrealistic dreams!
[Edited on 15-5-2016 by PHILOU Zrealone] |
You of course noticed that Glyme and Eosin Y write the same kind of équations with the same kind of mistakes... do you?
Glymes wrote
1) Acetic acid + tetranitromethane = trinitroacetic acid?
CH3COOH+C(NO2)4=C(NO2)3COOH+CH3NO2 (which would bubble out of solution.)
2) Ammonium chloride + trinitroacetic acid = dinitroaminoacetic acid?
NH4Cl+C(NO2)3COOH=C(NO2)2COONH3+HCl
So here again:
1°) Don't use =, use arrows
2°) The first equation may eventually make a messy media
CH3-CO2H <==--> CH3-CO2(-) + H(+)
C(NO2)4 <==> C(NO2)3(-) + NO2(+)
By crossed recombination
CH3-CO2(-) + NO2(+) --> CH3-CO-O-N=O (mixed anhydride of acetic acid and nitrous acid)
H(+) + C(NO2)3(-) <==> HC(NO2)3
This explains that C(NO2)4 (TNM) is sometimes used as a nitration media (but this is risky and making solutions of acetic acid and TNM is potentially
explosive because acetic acid is a fuel (yes 100% acetic acid burns).
3°) If your first equation was right; then CH3-NO2 would not bubble out of solution (with a bp of 101°C NM will not boil spontaneously under
atmospheric pressure and at lab temperature!
4°) If your first equation was right; then it would be CO2 that would boil off solution! For the reasons invoqued in previous posts carboxylic acid
with strong electron withdrawing groups decarboxylate easily...this is even more true when 2 EWG are involved and a fortiori if 3 are involved...
(O2N-)3C-CO2H ==> HC(NO2)3 + CO2(g)
5°) Your second equation is wrong and violate many laws of chemistry.
-For an obscure reason you take rid of one NO2 groups (dark matter again)
-For the same reason you take rid of one H atom NH4(+) turns into NH3 linked to a molecule
-trinitroacetic acid would make trinitromethane (+CO2); the first one would be a strong acid if it existed, the second is a strong acid but none would
beat the very high acidity of HCl (see pKa tables to understand)...and HCl would thus stick to the NH3 base... --> no exchange of NH4(+) possible.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Could you elaborate a bit?
E= m*c²  
you wrote E = 0*h*2
so E = 0  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
big Theory
Thank you for interest about my Great Theory of the water. O = oxygen H = hydrogen. 2 = multiplying, H x H = 2 pieces of Hydrogen. Well.. hm...So
much in my theory would better not much dissect. Is it international truth, it is clearly. Water know everybody. Please, do no drilling and do not
doubts. Everybody can forget on E = m . c 2.
Now apply E = O . H 2. Dot.... ...LL ...LL
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
This could be interesting:
70:25:5 NH4ClO4 almitic acid/napthenic acid/gasoline:magnesium powder. This might
act as a sort of 'vacuum' explosive: napalm uses up enormous quanitities of oxygen and this mixture could detonate and create a pressure wave that
collapses in on itself... almitic acid/napthenic acid/gasoline:magnesium powder. This might
act as a sort of 'vacuum' explosive: napalm uses up enormous quanitities of oxygen and this mixture could detonate and create a pressure wave that
collapses in on itself...
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
But the ammonium perchlorate in your mixture provides that oxygen.
So called "vacuum bombs" rely on large amounts of metal powder that combusts in the air after the detonation. First it heats the air and detonation
products to a few thousand kelvins, expanding them by a factor ten or so. Then the products condense and rapidly lowers the pressure. The low pressure
wave deals very nasty damage...
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
Ah, sorry about that. Fuel-air bombs can also work like this. They should be banned as weapons of mass destruction. That mixture could still be
interesting.
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Thanks PHILOU for your answer, I'll try it soon.
[Edited on 19-5-2016 by kratomiter]
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by kratomiter  | One quick question: I want to try the synthesis of guanidine perchlorate using ammonium perchlorate instead of perchloric acid.
NH2C(=NH)NH2 · ½H2CO3 + NH4ClO4 -> NH2C(=NH)NH2ClO4 + NH3 + CO2 + H2O
At high temperatures (>80ºC) in water solution NH3 and CO2 will leave the system, shifting the reaction to the right. Is this correct? Synthesis
of HClO4 is not very amateur-friendly and I want to avoid it. |
.
Guanidine perchlorate, however, is a detonation-sensitive material which is hazardous to handle. Its impact sensitivity by the Bureau of Mines Test (2
kilogram weight, 50 probability) is below 5 centimeters (cm.) The hazards involved in preparing propellants from it have therefore interfered with its
utilization.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
I think that aminoguanidine perchlorate would be more stable?
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
I think that aminoguanidine perchlorate would be more stable?
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Thank you for your warning ecos. It will be a little batch, just to see if it's useful as a cheap primary.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ecos  | Quote: Originally posted by kratomiter  | One quick question: I want to try the synthesis of guanidine perchlorate using ammonium perchlorate instead of perchloric acid.
NH2C(=NH)NH2 · ½H2CO3 + NH4ClO4 -> NH2C(=NH)NH2ClO4 + NH3 + CO2 + H2O
At high temperatures (>80ºC) in water solution NH3 and CO2 will leave the system, shifting the reaction to the right. Is this correct? Synthesis
of HClO4 is not very amateur-friendly and I want to avoid it. |
.
Guanidine perchlorate, however, is a detonation-sensitive material which is hazardous to handle. Its impact sensitivity by the Bureau of Mines Test (2
kilogram weight, 50 probability) is below 5 centimeters (cm.) The hazards involved in preparing propellants from it have therefore interfered with its
utilization. |
Only when dry! If the reaction is made in water...no risk.
Then slow evaporation in dry air at ambient T° to get dry GP.
It is indeed powerful detonating stuf.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Stop thinking that what you think is the truth.
AGP would eventually be more sensitive to shock and friction than GP because it contains a hydrazino group what reduces the energy (enthalpy) of
formation of the compound and increases its energy (enthalpy) of explosion.
About chemical stability both salts are salts from a strong base with a strong acid and are thus storage stable.
Typical example are NH4ClO4 and N2H5ClO4:
Despite NH4ClO4 is denser than N2H5ClO4...
N2H5ClO4 is more sensitive to shock and friction than NH4ClO4 and its VOD, brisance and Lead Block Test for 10gr are higher than those of NH4ClO4
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
..
36
37
38
39
40
..
81 |