| Pages:
1
2
3
4 |
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
What to do with chlorine gas ?
This weekend I hope to set up a generator of hot dry chlorine gas for my 'Dragon's Blood' synthesis (AuCl4)
so I am wondering what other syntheses I may use chlorine gas for while I have the generator set up ... ideas/suggestions ?
If my titanium arrives in time I may try TiCl4
but I can't think of any useful reagents to synthesize.
[Edited on 11-5-2016 by Sulaiman]
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
There is always sulfur dichloride and sulfur monochloride -- https://www.youtube.com/watch?v=oYw89ieffa4 I understand it is quite a bit of fun.
Some AlCl3 springs to mind. FeCl3 is also a possibility.
And all else failing, you can attempt bromine production by displacement with Cl2.
Maybe have a shot at ampouling some. If you have access to dry ice or even better, liquid nitrogen you could set up a little production run. A
couple of dozen of those sold on eBay should land a tidy profit.
As a side note, I had a thought on the AuCl3 synthesis. I have some gold leaf. I wondered about laying it on a piece of glass and putting it
directly in a stream of dry chlorine gas. I don't know (and haven't looked up) whether gold will react directly with chlorine or whether it is
necessary to go via chloroauric acid. But it seemed like a relatively simple set-up to attempt. One of the positive aspects if it works is that you
should end up with an even dispersion of the crystals on a microscope slide ready for viewing.
Thoughts?
|
|
|
TinSandwich
Harmless

Posts: 29
Registered: 24-2-2016
Member Is Offline
Mood: No Mood
|
|
What about making FeCl3? (https://www.youtube.com/watch?v=KRvqv5KQysg) It's like fireworks but toxic! Yay!
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Come to think of it, some anhydrous SnCl2 might be a useful thing to have on hand too.
|
|
|
RogueRose
International Hazard
    
Posts: 1597
Registered: 16-6-2014
Member Is Offline
|
|
S2Cl2?
|
|
|
Chem Rage
Harmless

Posts: 28
Registered: 28-3-2015
Member Is Offline
Mood: No Mood
|
|
Chlorine has so many different chemical applications. For obvious reasons, avoiding breathing it in and ensure very good ventilation. Whenever I carry
out reactions with Cl2, I do so on a very small scale using my smallest spec glassware. I also bubble unreacted Cl2 "exhaust" gas into a chemical
scrubber consisting of a concentrated solution of sodium thiosulphate, which reduces the Cl2 to harmless chloride.
Cl2 (dried) is useful for preparing anhydrous metal chlorides that are prone to undergoing hydrolysis if exposed to water or moisture. The oxidising
properties of Cl2 can also be utilised in the preparation of other halogens, except for fluorine.
[Edited on 11-5-2016 by Chem Rage]
[Edited on 11-5-2016 by Chem Rage]
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Putting hot aluminum in chlorine gas is a good demonstration. You could also chlorinate ethanol to acetaldehyde.
|
|
|
Eosin Y
Banned troll
 
Posts: 83
Registered: 8-5-2016
Location: Eton College science department
Member Is Offline
Mood: Aga needs to cool his heels
|
|
What are you using as a chlorine generator? Trichloroisocyanuric acid-hydrochloric acid?
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
I prefer the electrochemical method, gives me precise control of volume.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Stoichimetry also works 
I like a Balloon instead of an excess gas scrubber.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Do you have KI, NaI or KIO3 and one of NaOH or KOH? If so, then you could try to make the very interesting periodate salts:
http://woelen.homescience.net/science/chem/exps/KIO4_synth/i...
http://woelen.homescience.net/science/chem/exps/Na2H3IO6/ind...
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Bubble it through water; toss in some calcium carbide; watch them spontaneously combust when the bubbles merge.
Many videos of this seem to be rather careless in regards to their handling of chlorine; maybe you can make a better one:
https://www.youtube.com/watch?v=1QDGxY_oQVg
https://www.youtube.com/watch?v=1Y5aCSOSQgU
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
myristicinaldehyde
Hazard to Others
  
Posts: 166
Registered: 23-4-2016
Location: .͐͌ ͛҉̻̫̰̻̖E̮ͮ̐́̚ ̢̗̅̉ͩ͂̒̌.̯̻̺̯̀̎͂̄ͩ̚
Member Is Offline
Mood: сорок пять
|
|
Make silicon tetrachloride! Fun stuff.
It's used to make some more exotic, less eye-destroying silicon compounds, but it's cool in its own right.
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
WOW !
I'm overwhelmed, lots of reading to do. thanks to all.
I intend to use 15% sodium hypochlorite solution dripped into 36% HCl to generate chlorine gas.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Get DCCA or TCCA pool shock instead.
Much simpler.
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
Maybe, but I have most of a litre of 15% sodium hypochlorite solution in a hdpe bottle that looks a little like a balloon when in hot weather,
so I may as well use some before summer.
If my initial attempts are successful then I may make a YouTube video, in which case I may use TCCA to allow easier replication.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
When i tried it with bleach the foaming agents/surfactants frothed up like crazy.
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
aga
My 'bleach' was sold as sodium hypochlorite 14% - 15%,
I will test this evening to see if it foams.
I measured the pH at 12.3 so c1% w/w NaOH stabilizer.
I ordered some TCCA tablets last night since others may not have access to 15% non-foaming NaOCl, plus I've not tried TCCA yet.
mayko
The Cl2 + C2H2 looks fun and i have calcium carbide so that one is on the list.
The safety in those videos was adequate, very low volume of escaped chlorine gas.
woelen
yes, I must have a go at periodates, thanks.
Can I just use KI instead of KIO3 as on your website ?
plus, my titanium arrived yesterday so a little TiCl4 too.
I may even try freeing a little bromine from KBr.
that should keep me busy this weekend 
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Sulaiman  |
mayko
The Cl2 + C2H2 looks fun and i have calcium carbide so that one is on the list.
The safety in those videos was adequate, very low volume of escaped chlorine gas. |
I just posted a couple of books on chemical demonstrations: http://www.sciencemadness.org/talk/viewthread.php?tid=28848&...
The C2H2 / Chlorine reaction is in one of these books. (I think the second). Full procedure is given. It really is a lot of fun! But it is rather
energetic as you would expect.
[edit]
You seem to have been rather busy with your chem recently Sulamain. Lots of interesting ideas percolating in that brain of yours and some nice
experiments attempted. As always, I am looking forward to some more lab time. I hope you have a nicely chlorinated weekend. 
[Edited on 12-5-2016 by j_sum1]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Try mixing dry Cl2 and H2 absence any oxygen (exactly how to remove small amounts of water vapor and O2 may require some thought and experimenting).
The presence of oxygen apparently terminates the light induced H2/Cl2 chain reaction.
Also, no water vapor as this introduces unstable HOCl (from the action of Cl2 and water) which can decompose liberating some oxygen.
Use a uv light pointer to ignite the H2/Cl2 mix in a highly kinetic explosion (so small volumes only).
Videos available online. See https://www.google.com/search?sclient=tablet-gws&site=&a...
Safety precautions a must!
[Edited on 12-5-2016 by AJKOER]
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
Although the H2 + Cl2 + light is a tempting experiment,
I am avoiding experiments with significant explosions as I don't want to worry my neighbours or get any hassle,
as a teenager I made MANY explosions, all the neighbours knew it was me but did not complain,
but society is not as tolerant of such things now,
and in the current environment, if I thought a muslim neighbour was making explosives I'd be concerned too, and I'm a muslim !
I try to avoid lots of smoke or smell for similar reasons, even though my neighbours know of my hobby.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Sulaiman:
I agree with your comments.
The last time I made a chemistry related comments to a stranger, they ask me if I made drugs! I think of this as a societal consequence of the
'Breaking Bad' TV series.
What I really find scary with my background knowledge from having serving on a jury trial combined with my perception of a near total lack of science
education in the general population, is explaining to such 'peers' how making a small amount of HYDROGEN from water is not something so terribly bad
to rot in prison. Mixing H2 and CHLORINE, to make an even small bang, would be an even more difficult task to explain.
[Edited on 13-5-2016 by AJKOER]
|
|
|
Fegie
Harmless

Posts: 17
Registered: 6-4-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AJKOER  | Sulaiman:
I agree with your comments.
The last time I made a chemistry related comments to a stranger, they ask me if I made drugs! I think of this as a societal consequence of the
'Breaking Bad' TV series.
What I really find scary with my background knowledge from having serving on a jury trial combined with my perception of a near total lack of science
education in the general population, is explaining to such 'peers' how making a small amount of HYDROGEN from water is not something so terribly bad
to rot in prison. Mixing H2 and CHLORINE, to make an even small bang, would be an even more difficult task to explain.
[Edited on 13-5-2016 by AJKOER] |
Yup, when someone you dont know too well hears you do chemistry their immediate reaction is "oh prolly drugs and explosives!"
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
You can use KI instead of KIO3. You just have to add more KOH and have to bubble more Cl2 in order to get the KI fully oxidized to KIO3.
For each iodide ion you need 4 molecules of Cl2 and 8 hydroxide ions. Add a slight excess of hydroxide and bubble some excess Cl2, so for each mol of
KI, take 9 mols of KOH and appr. 5 mols of Cl2. That should give you the desired KIO4.
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
Slight delay ...
I had to order a 19/26 to 24/29 adapter to connect my drip funnel to an erlenmeyer for my chlorine generator.
I tried a different setup as my chlorine gas generator, but had a small leak.
My TCCA 20g tablets arrived yesterday, much cheaper than using my NaClO.
My 15% NaClO from APC does not foam with HCl by the way.
My current plan is to use four 1" x 6" side-arm test tubes with rubber bungs and glass tubing,
anti-suckback, drier, heated reaction vessel, scrubber.
I've dropped the chlorine gas pre-heating idea ... overly complicated heating arrangement.
For today I'll just do a little more gold recovery.
EDIT: a photo of my sketch of my proposed chlorine gas generator, nothing new, just making sure I'm not missing something important.
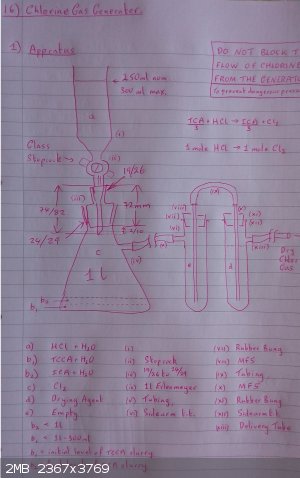
More editing;
d) the drying agent is CaCl2 prills
(i) is a D3/26 250ml drip funnel
I have ordered some pvc tubing as my silicone tubing is not suitable.
Not shown are afterthought pvc discs with a central hole as a protector for the rubber bungs.
[Edited on 15-5-2016 by Sulaiman]
|
|
|
| Pages:
1
2
3
4 |