| Pages:
1
2 |
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Germanium from Transistor
So, I recently acquired an old GE germanium transistor. These aren't used all that often, due to silicon being so much more widespread, but do contain
some significant amount of pure germanium.
Here's a picture of the transistor, after exposing the inner workings with a hacksaw:

It's a bit hard to make out from my blurry phone camera, but the iridescent base is a copper heatsink. In the middle there's a circle of metal that
might be indium (judging from what I looked up when I researched what's inside these things), followed by the rectangular piece of what I know to be
Ge, and finally another little blob of In (maybe).

This is a closeup of the square of Ge in the middle. It's maybe half a millimeter to a millimeter thick, and about 3/16" of an inch to a side. Smaller
than what I expected, but workable.
So, my question to you is: How should I get the Ge out? I read that it's resistant to acids, but I'm hesitant to use HCl or H2SO4 on it until I'm
really sure that it'll work.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Use its resistance to acids to your advantage.
Get the disc of Ge out as best as you can by mechanical means. Then dissolve any adhering base metals by subjecting the lot to HCl, to dissolve them.
Ge is very resistant to HCl and H2SO4 but will be attacked by HNO3 or AR, IIRW...
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
ec1, I was thinking of doing the exact same thing. Thanks for starting this thread.
Could you please repost the pix using the SM server. Tumblr is blocked on the work computer that I am using.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by j_sum1  | ec1, I was thinking of doing the exact same thing. Thanks for starting this thread.
Could you please repost the pix using the SM server. Tumblr is blocked on the work computer that I am using. |
I started with the biggest transistor I could find in hopes of getting a sizable chunk of Ge. If this is all that's inside, I shudder to think what
lengths I'd have to go to to get it out of the smaller ones, let alone the Ge diodes I brought home.
The photos are attached.
blogfast25, that was my initial plan, but I was hoping for some mechanical means. I wonder if this will also get me some indium chloride or sulfate I
can work with? Presumably not much, but still.
 
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
In the circumstances, the easiest way to check if the other stuff is indium is to see if you can scratch it with your fingernail. Indium is very soft.
It also has a very low melting point, which might hlp you remove the chip from its mounting.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
found some similar pics on google image search. pretty interesting, thanks for giving me something to look up 
lower portion of this page has some similar pics with description
http://www.radiomuseum.org/forum/history_of_telefunken_semic...
different style pic with arrows
http://ibm-1401.info/IBM083GeNPN-withComments-.jpg
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
An unfortunate update. While I was handling the transistor, I tried to get that wire leading off the top of the Ge plate off in a few ways. This
resulted in the Ge plate shattering, leaving me to (definitely not frantically) collect the shards of Ge:

So, on a positive note, I now have a sample (tiny as it is) of pure germanium, and I learned that you can take that wire off simply by gripping it
with a pair of pliers and pulling upward - it just comes off the Ge plate, not sure why.
However, this isn't a very good-looking sample of germanium. I do have a few other transistors - they're smaller, resembling 100 uF electrolytic
capacitors in both size and shape, and silver in color. The part number for these is 2SA353, for which I could find no datasheet (but the Internet was
very sure these were germanium transistors). Here's one below, and next to it is what's inside:
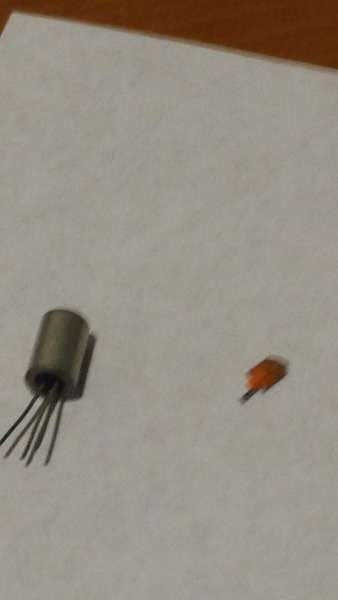
Inside appears to be a silvery metal plate, though it's hard to tell if it's Ge or some other metal - it's coated in some kind of orange plastic.
Apologies for the poor picture quality...

Who could possibly be this cruel.
Well... On another positive note, I won't have to worry about sample size if I can get that plastic off, as I have a few of these smaller transistors.
They're also quite easy to take apart - just saw off the top and wiggle the lead wires around a bit, and you can actually just poke them through and
out of the recently sawed-off hole in the casing.
Anyone have any ideas about getting that plastic off?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Again no visible pics. 
Can you dissolve the plastic in an organic solvent?
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Don't worry, you aren't missing much. What solvents are typically used?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ummm
One that softens or dissolves the plastic.
I use the scientific method for things like this:
Hypothesis -- organic solvent X that I have a bottle of somewhere will dissolve this plastic that I don't want.
Method -- attempt to dissolve plastic in solvent.
Observations -- confirm whether or not plastic has dissolved.
Accept or reject hypothesis based on observations.
Propose new hypothesis -- organic solvent Y that I own will dissolve this piece of plastic that I don't want.
Repeat until (a) a hypothesis is confirmed, (b) I run out of hypotheses or solvents, (c) it's dinner time or (d) Thomas Edison's* efforts** with
developing a lightbulb start to look sane.
*Quote=Edison
I have not failed. I've just found 10,000 ways that won't work.
**Quote=Tesla
If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found the
object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Why destroy such beautiful germanium transistors? The amount of Ge you get out of them is minute, while the transistors have quite some value.
Germanium transistors are getting rare and expensive.
If you want a nice germanium sample, then buy some off eBay. It can be obtained for three or so euros per gram, often without additional cost of
shipping:
http://www.ebay.nl/sch/i.html?_from=R40&_trksid=m570.l13...
A 1 gram sample already is MUCH more than the tiny specks you get from a germanium transistor.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by woelen  | Why destroy such beautiful germanium transistors? The amount of Ge you get out of them is minute, while the transistors have quite some value.
Germanium transistors are getting rare and expensive.
If you want a nice germanium sample, then buy some off eBay. It can be obtained for three or so euros per gram, often without additional cost of
shipping:
http://www.ebay.nl/sch/i.html?_from=R40&_trksid=m570.l13...
A 1 gram sample already is MUCH more than the tiny specks you get from a germanium transistor. |
Very true, but there's something to be said for 'making' a sample instead of buying it, even if 'making' it does consist of destroying perfectly good
circuitry. Gives it a bit of a backstory, so to speak.
To each their own, then.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
On the tangentially relevant subject of dodgy pictures, do people generally know that you can take a picture through a magnifying glass?
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by elementcollector1  | Very true, but there's something to be said for 'making' a sample instead of buying it, even if 'making' it does consist of destroying perfectly good
circuitry. Gives it a bit of a backstory, so to speak.
To each their own, then. |
Of course, if you want to do this, then it is your good right, no offence taken 
But . . . I do not consider this 'making' germanium, it is just taking the element out of its package. The package is a little bit harder to open than
the package around the germanium if you buy it off eBay, but in principle the action is similar.
If you really make and isolate germanium from an OTC product, then that would be very interesting.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ok. but other than transistors and diodes of a certain vintage, what OTC products contain germanium?
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I do not know of any OTC product containing germanium. My remark was more in a general sense. Maybe someone knows of some germanium-containing OTC
product.
BTW, a vintage germanium transistor also is not an OTC product. In the city where I live I do not think you can buy them anywhere, you need specialist
sellers or someone who still has some old stock somewhere. For me a germanium transistor is as (non-)OTC as a ready made sample of germanium.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | | Ok. but other than transistors and diodes of a certain vintage, what OTC products contain germanium? |
Old battery chargers Used Germanium rectifiers. Allot more material there.
http://periodictable.com/Samples/034.2/s7s.JPG
They are riveted to the back of the metal frame.
[Edited on 10-5-2016 by XeonTheMGPony]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by woelen  | I do not know of any OTC product containing germanium. My remark was more in a general sense. Maybe someone knows of some germanium-containing OTC
product.
BTW, a vintage germanium transistor also is not an OTC product. In the city where I live I do not think you can buy them anywhere, you need specialist
sellers or someone who still has some old stock somewhere. For me a germanium transistor is as (non-)OTC as a ready made sample of germanium.
|
I've seen germanium medicinal supplements around, do those count? 
Anyway, these were free to me because they were sitting in the back of our circuits lab, gathering dust. We're allowed to take any component as long
as we use it, and they have more, so it's not like I'm doing anyone else a grave inconvenience.
Upon looking at those germanium rectifiers, they are quite expensive. There'd probably be a lot of germanium inside, though, so this bears some
thought.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
I think you are getting confused with selenium rectifiers. 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Seconded.
Edit:
The very first radio receivers were the 'Cat's Whisker' type which used a lump of germanium with a very thin wire touching it.
If you could find one of those, the content of the element i struggle to spell would be much greater.
[Edited on 10-5-2016 by aga]
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Verry well could be, been a while since I had to work with them.
I'd love to get my hands on a Mercury arc rectifier.
[Edited on 11-5-2016 by XeonTheMGPony]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Quote: Originally posted by aga  | The very first radio receivers were the 'Cat's Whisker' type which used a lump of germanium with a very thin wire touching it.
If you could find one of those, the content of the element i struggle to spell would be much greater.
|
No, aga, you are also confused! 
These used the natural mineral galena, lead sulphide.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
You could us just about anything as a detector for a crystal set. Galena, pyrites and coke were common choices.
My dad used a rusty razor blade for one.
So it's possible that you could make one with germanium or you could be thinking of one of these.
http://rfelektronik.se/manuals/Datasheets/OA91.pdf
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | No, aga, you are also confused! |
So i am ! Funny, but i always thought it to be Ge.
| Quote: | | or you could be thinking of one of these |
I had about 20 of those off a circuit board from an army surplus store, many many moons ago.
Edit:
Bugger !
I jumped the gun there and confused the OA71 DIODE with the OC71 transistor !
Definitely losing it.
[Edited on 11-5-2016 by aga]
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
| Quote: | | I do not know of any OTC product containing germanium |
There are germanium supplements. Look for 'organic germanium'. http://www.luckyvitamin.com/p-17536-jarrow-formulas-germaniu...This one for instance claims 150 mg of a germanium compounds per capsule. Should be
a visible amount. Probably even more than you'll get from butchering rare transistors.
And a nice challenge to isolate it and prepare the elemental form.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
| Pages:
1
2 |