chemStudent9000
Harmless

Posts: 3
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Help me synthesize an interesting molecule
I am an organic chemistry student and in lab we have performed the attached synthesis to form 2,3-Dihydro-2,2,4-trimethyl-1H-1,5-benzodiazepine 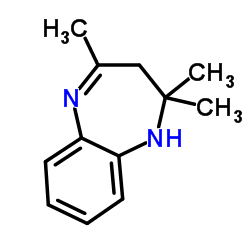 . I can probably provide the paper with the entire mechanism if anyone is interested. . I can probably provide the paper with the entire mechanism if anyone is interested.
Having performed this synthesis our next task is to come up with an idea for another molecule. This new molecule can be synthesized from the above
benzo product or we can alter the original synthesis in some way to get this new molecule.
The reason I am posting here is to see if anyone has any ideas on what I should synthesize. I assume most other students will make some random change
to their starting reactants to yield some molecule with little thought to their final product.
I would like to synthesize something interesting but I lack the chemistry knowledge to come up with much on my own. Are their any chemistry resources
out their were I can search for molecules with similar structure to the on above and get ideas for the new synthesis?
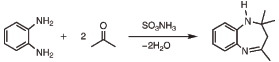
[Edited on 9-2-2016 by chemStudent9000]
|
|
|
Neuro-
Harmless

Posts: 29
Registered: 24-1-2016
Member Is Offline
Mood: No Mood
|
|
Well benzodiazepines are some of the most studied compounds ever (top selling, been around for decades. You could easily find resources on places like
rhodium due to the fact most benzo's are drugs, and there are a number of syntheses you could attempt https://www.erowid.org/archive/rhodium/chemistry/diazepam.ht... You might not want to make scheduled substances (prison wouldn't be fun) but you
could simply not substitute the wrong things in the right place (search the Markush structure). I'd love to see the paper, I've been looking for a way
to try benzodiazepine synthesis without the legal problem of making a scheduled substance. Personally making basic ring structure of midazolam without
the attached phenol ring and the halogens would be a cool exercise.
[Edited on 9-2-2016 by Neuro-]
|
|
|
chemStudent9000
Harmless

Posts: 3
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Thanks for the input Neuro, especially with the idea of searching for the Markush structure. I've attached the paper for anyone interested. I would
love to hear what ideas other people have.
Attachment: benzodiazepine supplemental information-1.docx (2.1MB)
This file has been downloaded 2040 times
Attachment: benzodiazepine.pdf (73kB)
This file has been downloaded 501 times
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
You might consider using ethylenediamine in place of the o-phenylenediamine. This could produce a diazepine instead of the benzodiazepine. The sterics
of the reaction would be considerably altered and could lead to an interesting discussion based on whatever products you might find.
AvB
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I don't know much about benzodiazepines ,so I don't know which are legal and which are not,so you have to check that out
a few things you could do would be to halogenate or hydroxylate the phenyl ring,alkylate the secondary amine or use halogenated ketones rather than a
plain one.(the logic being since trichloroacetaldehyde is a sedative,and benzodiazepines are also used as sedatives,something should happen  ) .maybe you could use dibenzylketone or even dibenzylidene acetone and cause furthur
resonance ) .maybe you could use dibenzylketone or even dibenzylidene acetone and cause furthur
resonance 
|
|
|
chemStudent9000
Harmless

Posts: 3
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
These are all great ideas, I'll have to think about what I want to do. I'll be sure to post back here and let everyone know what I come up with.
|
|
|