| Pages:
1
2
3
..
18 |
Xenos
Harmless

Posts: 22
Registered: 29-8-2002
Member Is Offline
Mood: No Mood
|
|
Sodium!
Ok, well my power supply came from ebay (5V @ 50amps)  so i tried some simple
electrolysis. Using carbon electrodes, as close together as possible i first tested it in a salt water solution. It bubbled rapidly, and i plan to
attempt to make and capture the Hydrogen and fill a balloon with it. I then proceeded to try KOH. I heated it on my hotplate and put the electrodes
in. Soon it began bubbling rapidly, and forming a kinda grayish mix. However, i stoped that experiment. Yesterday i tried to heat some NaOH, but my
hotplate wasnt getting hot enough, so i put a torch to the crucible and it began to bubble away. This happened for about 30 min, while the NaOH was
turning a silvery black color. ( I suspect this was the C coming off, and they were a bit smaller than before). However, my crucible got a hole in it,
so i stoped. That sat overnight, and i then wetted the mix, to find some whitish specks on the side got hot when water touched it. I think if any Na
or K formed, the water from the + electrode converted it back to OH as soon as it formed. Today i made a crucible with fire cement and put an C rod in
the center. Hopefully that will prevent anything from getting to the material. so i tried some simple
electrolysis. Using carbon electrodes, as close together as possible i first tested it in a salt water solution. It bubbled rapidly, and i plan to
attempt to make and capture the Hydrogen and fill a balloon with it. I then proceeded to try KOH. I heated it on my hotplate and put the electrodes
in. Soon it began bubbling rapidly, and forming a kinda grayish mix. However, i stoped that experiment. Yesterday i tried to heat some NaOH, but my
hotplate wasnt getting hot enough, so i put a torch to the crucible and it began to bubble away. This happened for about 30 min, while the NaOH was
turning a silvery black color. ( I suspect this was the C coming off, and they were a bit smaller than before). However, my crucible got a hole in it,
so i stoped. That sat overnight, and i then wetted the mix, to find some whitish specks on the side got hot when water touched it. I think if any Na
or K formed, the water from the + electrode converted it back to OH as soon as it formed. Today i made a crucible with fire cement and put an C rod in
the center. Hopefully that will prevent anything from getting to the material.
The ASPCA never liked Shrodinger, but they never knew he was a theroretical physicist.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I prepared some sodium metal this last Saturday myself. I placed 20g of NaOH in a ceramic crucible, and proceeded to heat it on a hotplate until it
melted. I inserted two electrodes, both graphite, and bubbling began at the anode. I left it unattended for around an hour. When I returned, the
crucible contained a greyish, silvery liquid, permeated with greyish-black lumps. I allowed it to cool, and poured in charcoal lighter fluid to
protect the sodium from oxidation. I found that the somewhat grey and faintly silvery solid was brittle when cold. It clearly contained a lot of
graphite impurity. I chipped some of it out, and saved it in a beaker containing charcoal lighter fluid. I then reheated the contents of the crucible
to about 100C; and poured as much of the liquid sodium as possible into that same beaker. Localized boiling occured for a few moments; then several
dull silvery pieces of sodium metal settled at the bottom. I allowed the crucible to cool, and rinsed it with cold water. There was several sizzling
sounds, and what was left of the heavily-corroded cathode disintegrated completely. The mass of the ceramic crucible fell from 73.5g to 72g over the
course of the experiment. The anode was not corroded to any noticeable degree. Next time, I will use a copper cathode, and will pour off the sodium
metal while it is still molten (immediately after electrolysis) into chilled xylene.
I weep at the sight of flaming acetic anhydride.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
speculating from my armchair
In an ideal world, DuPont would deliver ingots of alkali metal to our doorsteps for production costs + 15%, or we would all have sophisticated machine
shops and expendable hunchbacks to operate the apparatus that we built. In the real world, I have never been able to confirm anybody's home production
of significant quantities of such metals. I recall reading somebody's old Usenet posts that claimed the writer had build some fairly good apparatus
and didn't have nearby neighbors, and so was able to produce kilogram-range quantities of potassium and sodium from the chlorides. Wouldn't I like to
do that.
With alkali electrolysis the main problem (apart from atmospheric isolation) is (IIRC) be protecting the metal from the products produced at the
anode, which dissolve in the molten alkali and can re-oxidize the metal. Unfortunately, this process is so old that references to its specifics are
difficult to find.
I would like to try using an equimolar mixture of NaOH and KOH, which is a eutectic melting around 200 degrees C (lower MP should significantly
increase ceramic crucible lifetime). The resulting Na/K alloy may have an unmanageably high reactivity and low melting point, though.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I think if you were to electrolise that when molten, firstly all the sodium would be produced because of it's higher electronegativity and lower
reactivity.
This will ofcourse have its effect on melting point, because the mixture will shift to Na + KOH.
Na/K alloys are often liquids at roomtemperature and very reactive, that is more reactive than pure sodium or potassium metal.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
trinitrotoluene
Hazard to Others
  
Posts: 142
Registered: 17-10-2002
Location: California
Member Is Offline
Mood: paranoid
|
|
I think what can be done to protect the K , Na alloy is do to pour a layer of mineral oil on top of the mixture and then do electrolysis.The only
problem is what kind of mineral oil has a boiling point of above around 200*C? I also consiture to do the same with Na metal.If the mineral oil can
remail a liuqid and not boil. If theres a hydrocabon that remains a liquid at the melting point of KOH then I guess it can be used to protect the
reactive metal.
TNT
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Sodium Ive done in small amounts without a blanket, and the air oxidation was amazing to watch, the metal constantly had a yellow aura around it.
Small hydrogen explosions tended to propell sodium across the room and this is something of a hazard. I used a copper cathode and a steel
anode/container, this seemed to work quite well.
Since I tried this, about 10 years ago, Ive picked up a fair amount more information from a number of sources.
The most common mistake is to overheat the hydroxide, in which the sodium is soluable. Too hot and the hydroxide/sodium produces a metaloid which
conducts electricity and electrolysis stops. Numbers from a book on sodium were, 5 degrees above the melting point of sodium hydroxide you get an 80%
yeild on a current basis, and 25 degrees above it you get nothing.
This partially explains why a lot of early references dont use gas burners to make sodium, they rely on the current itself to do the heating, and
controlling the current gives much more precision in controling the temperature of the cell.
vulture has a very good point about NaK alloys. This are much more reactive than either of them alone and burst into flames on contact with air at
room temp. The general concensus of the people Ive talked to, is that the difference in electrode potential is too small to produce only sodium from
a molten mixture of both salts. This is potentially very hazardous.
The book recommends I think 12% of carbonate in the mixture for optimum yeilds, and suggests a 50:50 mixture of sodium hydroxide with sodium sulphide
to reduce the melting point. This is aparently patented. Ive found information on hydroxide/nitrate/nitrite eutectics, but its impossible to guess
how reduction/oxidation at electrodes would affect the bulk mixture. Something that also bothers me about sulphide mixtures.
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
I saw in some chemistry manual the construction of cells for Na production from NaOH, and the Na is produced under some sort of bell-shaped thing.
Actually the (-) electrode it's inside of that bell. In this way, because sodium it's lighter than NaOH it'll float over the molten NaOH, under the
bell, and it'll be protected in the same time from the air. It's verry simple.
|
|
|
menchaca
Hazard to Self
 
Posts: 80
Registered: 12-3-2003
Member Is Offline
Mood: No Mood
|
|
Na with Hg catode
first of all sorry for my english
Ill try to explain as well as i can
i ve never try this but i think it can work
the idea consist on a quicksilver catode
where the Na forms an amalgam. After the quicksilver takes all th Na that it can (i dont know how long must it take) just take the amalgam and heat
it until Hg boils when Hg desapear solid Na should be there you can use an alembic to recover the Hg i´ll try to send you some draws of the idea i
had, you can send it to others and they will be able to tell you if it works or it doesnt and improve it
|
|
|
It burns
Harmless

Posts: 1
Registered: 14-3-2003
Location: Aberdeen (UK)
Member Is Offline
Mood: No Mood
|
|
hot electrochemical sodium
| Quote: | Originally posted by trinitrotoluene
I think what can be done to protect the K , Na alloy is do to pour a layer of mineral oil on top of the mixture and then do electrolysis.The only
problem is what kind of mineral oil has a boiling point of above around 200*C? I also consiture to do the same with Na metal.If the mineral oil can
remail a liuqid and not boil. If theres a hydrocabon that remains a liquid at the melting point of KOH then I guess it can be used to protect the
reactive metal. |
Im new and possibly wrong but couldnt you use tar, its got a realatively high melting temperature and is a hydrocarbon (at least im pretty sure it is)
might be a pain to get the everything back out of though
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Mencacha, the quicksilver amalgam becomes solid when the Na content goes above 0,7% and the maximum obtainable is 5% IIRC. Add to that that
you're going to need large amounts of Hg which is very expensive.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
When you increase the percentage of Na in Hg due to electrolysis , the emk increases and about 0,6-0,7 % Na, it will be the same as for the
H2O=> H2 + OH- reaction , thus it will not absorb any more Na by electrolysis but only decompose water.
So from 1 kg Hg(which is very expensive and hard to get ,lucky me that I have it you will recieve about 5 grams of Na.
you will recieve about 5 grams of Na.
The to evaporate this amounts of Hg I can imagine the difficulties setting up an adequate distillation arrangement.
/rickard
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I find it interesting that you refer to mercury as being very expensive. I can order it for about $30/kg, which makes it moderately priced to my way
of thinking. That's still an outrageous price compared to what it would cost in bulk, but not so bad compared to many other laboratory chemicals.
It seems strange that chemical availability and pricing varies so much by region, when you would expect these commodities to have near-identical
prices without geographic differentiation.
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
Hmmm...30 $/Kg is very cheap. Is it a regulated chemical ? In my country is banned and you can go into prison if you are caught with it. The price on
the black market it's said to be around 1000 $/kg.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
No, the personal possession of mercury is not regulated here, though I am sure it is illegal and punishable to dispose of it improperly. That does
explain the price difference, though. Commodities can become very expensive when they are not legally available.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Sodium!
Everyone, do yourself a favour and make do without NaOH. How? Well, just take salt with a bit of soda to make a low-melting eutectic (600 Celcius). No
corrosion - no water evolved - no trouble getting NaOH.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
NaOH has a much lower melting point, secondly, electrode corrosion is very high at 600C and you're dealing with Cl2 vapors.
Besides that, you always give me the impression of being a smartass when posting. Maybe you should calm down a bit and read the threads first before
you post something.
|
|
|
jimwig
Hazard to Others
  
Posts: 215
Registered: 17-5-2003
Location: the sunny south
Member Is Offline
Mood: No Mood
|
|
industrially mercury has historically been used to produce chlorine from NaCl acting as the anode (?) for a Whorill cell.
I think it goes like this
Na Cl electrolitically decomposes to Cl as a gas and Na as an amalgum with the Hg acting as an electrode on the bottom of the cell.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Castner Tiegel
NaOH sodium electrolysis is done with this:

The trick is the iron net between the electrodes (cathode - copper, anode - nickel) which are only 2cm apart. This is a very tight net (100/per cm*cm)
and yes it divides also the voltage of about 4V.
If interest I can post more data on this.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Sodium
I think a graphite electrode would be resistant to chlorine, as it's hard to get chlorine to attack carbon.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
I would say the mercury amalgane process is indiscutable for home use - the separation of the amalgane is plain to dangerous. (I don´t say this
often, yes?)
The NaCl electrolysis has in my eyes no advantages over the NaOH electrolysis. The "Castner Tiegel" shows the principle for how to setup a
electrolytic cell for the NaOH process. Not so diffficult. Materials are common and cheap.
Now I answer all the questions nobody has asked:
Pot: Iron
Cathode: Copper
Anode: Nickel
The distance between anode and cathode is only 2cm. The amperage at the cathode is about 1,6Amp/cmxcm at the anode about 1,1Amp/cmxcm. (Smaller units
call for higher current density). The voltage should be about 4V to 5V then, the current density is the more important point.
The magic part:
Between anode and cathode is a iron fabric as diaphragm which has about 100 mesh/cmxcm. This works naturally as a voltage divider. The fabric is fixed
in a way that 40% of the voltage are on anode - diaphragm and 60% are on cathode diaphragm.
The maximum temperature is 330°C, 20°C higher as the melting point of NaOH.
Suggestions: The use of steelwool between glassfibre fabric or glassfibre fabric with a steelnet embedded would do for a diaphragm. The better this
is the higher will the purity of the Na. I believe the voltage divider function being essential and that the ratio may shift to 30/70 instead 40/60
but not 50/50. The distance of diaphragm - anode/cathode IS critical.
The sodium from NaOH electrolysis is also cleaner as the one from NaCl - simple sedimentation is all whats needed. For even higher quality filtration
through an ironfabric or glassfibrefabric under inertgas or petroleum is used. (petroleum is not preferred - fire hazard). Remelting under paraffine
is another possibility.
The diaphragm as voltage divider and the bell on top - thats it. This sounds doable in a safe enough way to me and some steelwool for a improvised
diaphragm is sure for everybody available without loosing the improvised touch.... 
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
holla...
The thread is on sodium, so a photoreactor is perhaps not the right picture.
I apologize
<sniff>
Downs NaCl Sodium Production Cell
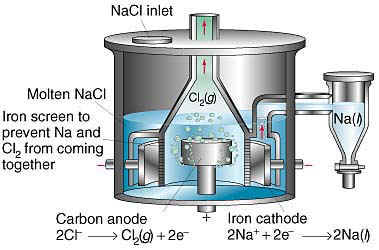
this is on topic I guess and answers what theoretic suggested: Yes! Graphite electrodes are useable in this process! The principle is the same working
with an iron diaphragm. I would suggest to exchange anode and cathode for to be able to harvest the Na central what seems much better for a smaller
scale unit. But I would fill it with NaOH anyways - no chlorine and much lower temperatures - no question.
|
|
|
blindreeper
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Does anyone who is good with pottery wanna try to make one of these out of ceramic? I imagine it would not be too difficult. If i had a kiln
and some clay and a wheel thingy I'd try it.
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
pottery usually contains SiO2 wich would be attacked by the molten NaOH. Except from precious metals like Pt, I believe that Ni is fairly resistant to
molten NaOH.
Ordinary stainless steel is corroded by lye above 60 C @ 0,1 mm/year, @ 100 C corrosion is about 3-4 mm/year so I expect molten NaOH @ 400 C would
show significant corrosion on stainless. But if it was hi-Ni content perhaps?
I can se a problem with the cathode , where Na is formed. the anode reaction would probably also be under quite reductive conditions since H2 is
evolved ?
OH- => H2O + H2 + e-
/rickard
|
|
|
kryss
Hazard to Self
 
Posts: 77
Registered: 11-7-2003
Location: N Ireland
Member Is Offline
Mood: No Mood
|
|
anyone ever try using a carbon fibre elctrode - should nbe shapable.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
ehem..
cathode = copper
anode = nickel
pot = iron
diaphragm = iron net, 100mesh/cmxcm
for NaOH electrolysis.
Thats the material used in industry and as it is cheap and easy I strongly suggest to use these materials also - whats wrong with them? Every
scrapyard provides a pot of thick iron for near free, the nickel willl be the hardest to get as I believe but far from impossible.
The "Castner Tiegel" is more on the point as the more beautiful second graphic!
Also thick iron will stand the molten NaOH for a long time there is a trick: heating from the center has the effect that NaOH solidifies on the bottom
and the walls of the cell. This protects from corrosion and contamination.
So preheating by a propane burner to about 290°C and then over the melting point and holding the actual temperature of maximal 330°C (400°C is far
out) by resistance/thermoelement inside the cell.
Not bad, isn´t it?
The hydrogen formed together with the Na provides the inert atmosphere over the Na. No problem - a feature.
If you want problems here they are:
- A pain in some bodyparts is the exact fixation of the diaphragm - I suggest strongly to use wider space between anode/cathode (more as only 2cm) and
compensate by higher voltage. This provides a good part of the needed heating this way - two with one.
- OTC NaOH is dirt like shit often. Big bad surprise! Use labgrade or test before use a small sample - cleaning the cell is - yes what? A work for
vulture? 
|
|
|
| Pages:
1
2
3
..
18 |