pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
weight of gold leaf from thailand
hi, someone weighed a leaf of gold in leaf/"foil" tipical sold in ebay from thailand?
4x4 cm - 24k
exist calculators but the tickness is unknown and my balance is not enough precice. doubts...
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I have much the same question. My scales are also inadequate. And I don't want to throw away the interleaving paper to weigh several at once.
I recall reading somewhere that typical gold leaf is 500-1000 atoms thick. I haven't done the calculation but that puts you in the ballpark. If
someone has some actual measurements i would be interested.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
If you do not mind destroying a typical leaf, you should be able to determine the weight... This is a CHEMISTRY forum, after all!
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Why don't you ask the seller?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
asked and reply with around 33gr with the paper 
Science is the religion of matter. It worships matter.
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Bert  | | If you do not mind destroying a typical leaf, you should be able to determine the weight... This is a CHEMISTRY forum, after all!
|
You are undoubtedly right Bert.
However, as far as my home lab is concerned, I am just starting out. I don't have anything much in the way of titration equipment. I have limited
reagents. My most accurate measuring device is my set of scales that read to 0.01g but I know the leaf will barely register. And my experience and
knowledge of gold chemistry is minimal.
I do have 50 leaves of gold at 3.5cm square. They are really delicate to handle and I have kept them with their interleaving paper. I wouldn't mind
knowing exactly how much I have got before I start doing anything serious with it. Of course I don't mind sacrificing a few to find out.So the
question raised is one that I have also had and is, I think, a good one. Has anyone had any experience with making a measurement? Has anyone got a
suggested approach that might work for me?
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
I'm somewhat curious as I have seen many of these Thailand gold leaf packets for pocket change... Have you tested any of the sheets to see if they are
in fact real gold? Perhaps simply submerge a piece into nitric acid to see if a reaction occurs, since many of the imitation gold leafs are made of
copper nitric acid should provide a very quick and decisive way of testing if it is in fact real gold.
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
No HNO3 in my supply yet. So no testing. There is a price difference between the products that are obvious Cu and the others. They do look different
too.
Thailand makes a lot of electronic. Most of the world's HDDs are made there. It doesn't really surprise me that there is some gold leaf around and
that enterprising individuals are flogging it off on eBay. In the context I would guess that the fake stuff is less readily available. "Imitation gold
leaf" is seldom of thai origin.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
If the weight is so small, why is the gold content important?
The price of the material is then probably dominated by the cost of manufacturing and handling.
Or do you just need to distinguish gold/copper?
In that case, you could also expose to FeCl3 or other etchants sold for etching circuit boards to try and dissolve copper.
Or do a flame test, copper gives a green flame (blue in the presence of chlorides).
During a course on analytical the teacher had us determine the gold content of Thai gold leaf he brought home from a holiday as an excersize in atomic
flame absorption spectrometry. When the gold content came out as 0, he initially told us we did something wrong and to redo it, but eventually the
evidence from our and other techniques convincingly showed it was some kind of painted foil with no actual gold.
[Edited on 29-10-2014 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
The gold was purchased for element collection. It needs to be reasonably pure for the same reason that any of the samples do. I am not going to
substitute nickel for cobalt or bottle some air and call it fluorine.
Since buying it I have decided to present gold in a few different ways. These include dissolved in aqua regia and colloid suspensions.
Probably there will be heaps of interesting things to do with the remainder. I believe gold chemistry is rather interesting. Now there is nothing
urgent here. But since the question was asked, I am interested in suggestions on how to measure what I have.
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
gold leaf tests - or beware Thailand
Hi to all.
I have been having difficulty with my Thai gold leaf dissintegrating when electrolysed, and changing colour when covered with epoxy.
So... I placed a piece in 32% HCl, brick cleaning grade. It dissolved to a yellow solution.
Hmme, methinks, I doubt that there is any nitrate in that.
I took a sample of this, approximately 5ml, and boiled to approximately 0.5ml, to remove HCl.
Hmme, it should turn pink in the presence of heated trisodium citrate, as colloidal gold is formed. So I made it back up to 5ml , and a clear
solution resulted. When this was boiled down to 0.5ml, I again had a yellow solution, but no sign of gold colloid. A repeat of the process, (made the
same residue up to 5ml, then boiled down again) led to a yellow solution, and white dried precipitate on the tube, but nothing indicative of gold.
A test on this solution with a "half pea" sized lump of sodium metabisulfite did not produce the snowstorm of glitter that I was expecting - or any
glitter for that matter.
Hmmme, I though, very interesting.
Treatment of the dissolved liquor with aluminium foil also did not produce any gold, but it made a cloudy purple solution, kind of like dissolving tin
in concentrated HCl.
At this point I tried my other, so-called pure leaf, and it did the same.
From this I conclude that I have been "had", and that ebay gold leaf must be viewed with extreme caution, even if advertised as pure or 24 Ct.
I can, however endorse Steve, from Gold Leaf Supplies
His platinum leaf was real platinum, and when suitably supported made a useful electrode. I am going to have to see him about some gold .....
| Quote: |
"Steve
goldleafsupplies
For all your gold leaf, silver leaf and gilding supplies
TEL: +44(0)1656 720566
FAX: +44(0)1656 729837
WEB: www.goldleafsupplies.co.uk" |
Cheers,
H
[Edited on 24-1-2016 by Harristotle]
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Hi Harristotle,
I am interested in hearing about your method for making electrodes from leaf foil metals.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Colloidal gold may be purple.
Some pregnacy tests are based on agglomeration of the purple colloidal gold into larger blue colloidal gold globules...
Here is a beautiful picture of colloidal gold colour variations
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Yes Philou, but no. I don't believe you.
So this calls for an experiment to test the idea.
The aluminium only turns it purple when there is a load of HCl about, otherwise nothing.
I am at this moment dissolving another leaf, and I will then produce some nano gold by spiking it with some 1% gold chloride solution I have.
If I can make colloidal gold in the tube containing 1% AuCl3 and dissolved "gold leaf", but not in the dissolved gold leaf alone, would this
acceptable prove that my gold leaf is a fake?
Thanks for the discussion, you inspired me to test more thoroughly, with a better control.
Cheers,
H.
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Making Colloidal gold
Here is a write up I presented on making colloidal gold. It was done so my kids could do some nanotechnology, rather than just read about it.
Note that the insight in this is the gold chloride supplier. Gold St Studios in Australia were just the best. This whole synth cost about $2 per run.
Lab to make gold nanoparticles
Materials
• 1% gold (III) chloride solution (gold chloride 1%. Obtainable from Gold Street Studios, 700 James Lane, Trentham East Vic 3458. www.goldstreetstudios.com.au)
• Citric acid (MacKenzie’s brand or similar, from Woolworths)
• Small 5ml measuring cylinder
• 50ml measuring cylinder
• 100ml conical flask or beaker
• Aluminium foil (6cmx6cm square)
• Bunsen burner, tripod and mat
• Matches
• 2 test tubes
• Laser pointer (any colour, under 1mw, teacher to control)
• 2x clean test tubes
• 1x dropper bottle of 10% NaCl
Method
1. Measure out the 0.6ml of 1% gold (III) chloride and dilute it to 20ml, using a 5ml and a 50 ml measuring cylinder. Transfer into a small conical
flask (100ml) or a beaker (100ml).
2. Make a cap out of aluminium foil, and cover the beaker or conical flask to minimise evaporation. Gently heat on a Bunsen burner and mat until you
have a rolling boil.
3. Measure out 2ml of 1% trisodium citrate.
4. Using heatproof gloves or tongs, swirl the boiling gold (III) chloride and at the same time, add the 2ml of trisodium citrate. Keep swirling, and
return to the low heat Bunsen. Make sure that you have replaced the aluminium lid to avoid evaporation.
5. Wait 10 minutes while the colour develops, then remove the flask and cool on your safety mat. What colour is the mixture now?
6. If you have one, shine a laser pointer through the beaker. What do you notice?
7. The colour of the solution is affected by the spacing of the particles in it. It is known that certain halides (eg chloride, iodide, bromide)
alter the spacing between particles in the solution.
8. Transfer your mixture into two test tubes. Into one test tube, add 10% NaCl solution dropwise.
9. What do you observe?
References
This practical was influenced by the following references:
Australian Government, AccessNano project (2009): Guide experiment 3: Making Gold Nanoparticles
http://education.mrsec.wisc.edu/nanolab/gold/ “Citrate synthesis of gold nanoparticles”, accessed 10/01/2016
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Thin sheet electrodes - gold
Purpose: Make a thin film electrode on glass so that the redox potential of a muddy environment can be read at depth.
1) vigorously clean a 25cm x 5cm sheet of glass. An old photo frame from an op shop (thrift shop) is a good source. Detergent, clean water, acetone,
dry.
2) Mix up a small batch of epoxy resin. Use a cotton bud to paste the thinnest possible smear on a region of the glass just larger than your gold
film.
3) using a paint brush, transfer your gold film to the epoxy, avoiding bubbles underneath.
4) Tamp down the electrode very gently with a tissue, and let dry.
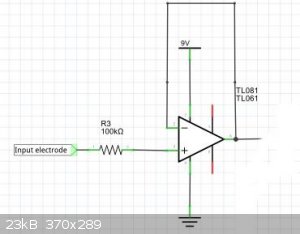
5) "dead bug" a T1081 op amp to make a unity gain non-inverting amplifier. Rationale: You want a high current version of the electrode potential so
that you can send it up or down a wire. You need as short as possible wire lengths until the op amp has done its magic.
6) Run a power, signal and ground bus down the glass using copper tape - the kind that UK nurseries sell to keep snails out of plants eg http://www.ebay.com/itm/1X-Single-Conductive-Copper-Foil-Tap...
Use conductive silver paint to connect the pins of various bits to various bus lines.
7) Cover the electronics, the bus lines, and the signal return lines with epoxy to shield them from the watery environment of the mud.
Power from at least a 9v battery. You should get about 4.5v output with no signal on electrode.
Note that each op amp at each level will have its own drift characteristics - the whole electrode array will need to be calibrated before use. (ie one
will give 4.5v, another 4.55v, another 4.6v depending on individual op amp characteristics). Put a Ag/AgCl electrode as a reference, and you should be
able to measure the Eo at different levels in the mud - these TL081 have a huge input impedence (10^12 ohms), so need very little current on the
input. This means very little disturbance of the electrochemical environment around the gold leaf, which, having such a big surface area should be
fairly robust)
I don't have pictures at the moment, and I'm very short of time, so I'll just leave this here for the moment.
Here is a picture of the electrode array, part way through construction. Both power bus's are present, but no electrodes are wired in. You can see how
my "gold" is corroded -not gold at all!
[bad img]http://www.screencast.com/t/J6KtxaW87e[/bad img]
Hope it is of interest.
Cheers,
H

|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
update on gold leaf
a single piece of gold leaf (2.5cm x 2.5cm -ish) was dissolved in approximately 10ml conc HCl.
It was boiled down to ~ 0.5ml, trisodium citrate was added, boiled and no purple or red colour was observed. A positive control "spike" of a drop of
gold chloride was added, boiled, and no change was observed.
To eliminate the possibility that carried over Hcl was interfering with the reaction, another test tube was filled with approx 10ml Hcl gold digest,
and boiled to dryness. This was then made up to 10ml with distilled water, and 2ml Trisodium citrate was added. No colour was observed on boiling. A
positive control of 1 drop of 1% gold chloride was added, and with continued boiling, turned purple, as would be expected from colloidal gold.
From this, I can't eliminate that the gold film is not gold (maybe the evaporated digest did not contain gold chloride, but some other form of gold).
But ... the gold metal completely dissolves in HCl (gold is inert to conc HCl, and doesn't produce colloidal gold under conditions where AuCl3 or
HAuCl3 do). And the metal reacts with epoxy resin! I think I have enough on this to say that the metal sheet is not gold. What do others say?
Cheers,
H
|
|
|
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I know that very fine gold dust from gold refining does not dissolve in HCl
so what you have is not pure gold.
It could be a very thin gold coat on some other metal foil ?
|
|
|
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
is 24k Au, at least my. even though the seller only have positive votes for a lot of people around the world buying the same Au doing quality items
and food, when in my hands the first thing I do is put a leaf random picked in conc. nitric and no dissolution, no color change. tai people have karma
fears, is a good friendly people, and a lot of this leaf Au end in Buda defaced effigies due to the quantity of gold leaf smeared over, and I never
see any of this Buda with a colorful chemical reaction rainbow from fake Au. 
If you can swallow down, working with leaf Au I think a lot of different results end if you make test when the Sun is in Leo or opposite, wit/hout
Moon etc 
wow!!! I just invented a new chem branch, tell him "AstroChem" :-DDDD
Well, sure someone else had already thought about this.
|
|
|