Craig
Harmless

Posts: 8
Registered: 4-5-2005
Member Is Offline
Mood: No Mood
|
|
Mercury and Aluminium
Hi,
I've been experimenting with mercury and aluminium. Some websites say that a violent reaction will take place when mercury comes into contact
with aluminium, but sadly this isn't the case  . .
Here's some photo's of the results:
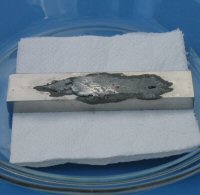 
 
 
To get the process started I need to use gallium. I always end the process by applying water. I think the gallium only helps the mercury when it is
in a liquid state. In the above images the gallium was only a liquid for a short period of time. Sometime soon I plan to do more experiments while
keeping the gallium in a liquid state. I also plan to use alternatives to gallium.
You can see the fullsize images, as well as others, on my website:
http://www.craigsarea.com/hg_al.html
The page is still under construction, so some of it is missing.
Hope you find it interesting  . .
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Very interesting. I would have thought that more damage would have been done to the aluminum, as Theodore Grey preformed about the same thing with a
larger block and had very fast and complete results. Different aluminum, maybe?
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
What is it supposed to do?
-rlr
[Edited on 11-5-2005 by runlabrun]
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Something like this. IIRC, the beam 'rusted' half away in 1.5 hours.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
His beam also ended up brown, too... I don't see how that happened.
Must be alloy has something to do with it - numbers 1199 (4n pure), 2024 (alloyed with copper), 7075 (zinc) and 320 and 390 (silicon) might be worth a
shot.
I bet you high silicon (390 is 20%) leaves a grainy fluff behind. 
Tim
|
|
|
BromicAcid
International Hazard
    
Posts: 3255
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
As has been mentioned elsewhere on this forum and in other places with straight mercury the reaction is fairly slow, but the addition of a small
amount of 'corrosive sublimate' speeds it up greatly. I believe John WW mentioned it.
|
|
|
Craig
Harmless

Posts: 8
Registered: 4-5-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | | What is it supposed to do? |
If you do a google for mercury and aluminium you'll find loads of websites that talk about the mercury attacking the aluminium. Some even say a
violent reaction will take place. You will also find a lot of contradictory results. But I noticed there are very few photos of the process. I just
wanted to see what happened for myself.
| Quote: | | Very interesting. I would have thought that more damage would have been done to the aluminum, as Theodore Grey preformed about the same thing with a
larger block and had very fast and complete results. Different aluminum, maybe? |
Theodore Grey was the one who recommended using the gallium. In his video you can actually see the gallium at the start.
From what I've seen, it seems that the gallium only helps when it is in a liquid state. While the gallium is a liquid it mixes with the mercury,
but when it turns solid it seperates from the mercury. Maybe in Theodores video he kept the temperature high enough to keep the gallium in a liquid
state throughout the whole of the process. In my experiments the gallium was turned to liquid by heating it, but the heat wasn't continually
applied, so it quickly turns back to a solid a few minutes into the experiment.
My experiment starts off like Theodore's, but doesn't go for as long. It effectively stops or slows right down. And I think this is
because of the gallium, but I haven't yet double checked.
| Quote: | | His beam also ended up brown, too... I don't see how that happened. |
I'm wandering if that's due to the gallium. If you pour liquid mercury onto liquid gallium you get a dirty greyish colour. Maybe the dark
colour, or the brown, is actually from the gallium. But that's only a guess, so I might be totally wrong  . Certainly if you handle gallium you get dirty hands . Certainly if you handle gallium you get dirty hands  . .
I've got some alternatives to gallium, which I'll try soon. It'll be interesting to see if the brown still appears when the gallium
isn't used.
| Quote: | | I bet you high silicon (390 is 20%) leaves a grainy fluff behind. |
The heat sink left a grainy light fluff behind whereas the bars left more of a powder. Could heat sinks have silicon in them?
I've asked a few aluminium companies if you would like to donate some aluminium samples, but none have replied yet  . The trouble is I don't know the precise properties of the aluminium I'm
using. If I had all the different types, along with their properties, I could do more acurate experiments. . The trouble is I don't know the precise properties of the aluminium I'm
using. If I had all the different types, along with their properties, I could do more acurate experiments.
[Edited on 11-5-2005 by Craig]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
As BromicAcid said, I'd suggest you make some HgCl2, this is much more effective in attacking aluminium because the Hg is generated in intimate
contact with the aluminium (be careful though, wear gloves!).
Metallic mercury has great trouble to amalgamate with aluminium, because of the oxide layer. Some HCl might help to remove it (but it forms again in a
split second, so first drip some HCl on the aluminium and then apply a drop of mercury while the HCl is still fizzing).
You should be able to get it to work without gallium!
[Edited on 11-5-2005 by garage chemist]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
If worse comes to worse I could send you some samples from my aluminum pile. Or vice-versa, someone could send me mercury (which I wouldn't mind
 ). ).
Heatsinks may or may not have silicon in them - depends if it was extruded or cast. If it's mostly fins arranged in one direction, especially
with serrated surfaces, it's probably extruded. Let me put it this way - can the item be cast between two or more mold halves, or would its
shape make more sense for it to be extruded then machined?
Tim
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
Its not really the need for Ga or Hg on Al.
Its the need for a liquid metal who's phase diagram shows intermetallic phases with the metal to be "attacked", which has a lower MP
than the base metal, and there is heat or mechanical stress applied.
With the application of stress to the base material, the liquid metal can run through the boundaries and simply seperate the crystals. Thats all it
does. It causes the crystals to come apart and sometimes be attacked by air. While Al has its awesome Al2O3 barriar, the liquid doesnt care once its
through (since air is still not able to penetrate to create the new Al2O3). Once in the metal it can break apart the crystals such that air can
penetrate the pours after the liquid has left the area. The grey junk you see is the Al2O3 coating the little crystals.
I have several samples on my desk showing LME in Cu/Fe, Ga/Al and Ga/Cu. you can spread the LME directionaly by application of stress in the right
places. try getting the Ga (I would never use Hg...) on a warm piece of Al following the steps below, and stress the Al by slightly bending it. If you
use Hg, wear gloves... at the very least)
for the low MP metals to be used for LME, you dont need to mix the Ga and the Hg... or have the Hg as HgCl. All you need to do is place the molten
meltal on the surface of the metal, and use a paper clip or knife to cut the surface of the metal to be 'attacked' from inside of the molten
metal. For example... Ga on Al. Warm the Al piece such that the Ga stays molten(rather than Al getting rid of the heat needed to keep the Ga liquid)
Place a SMALL blob/dab on the surface of the Al. Insert a sharp metal object (that has a hardness greater than Al) into the Ga blob. Scratch the Al
surface, to penetrate the Al2O3 layer. Air will not be able to get to the Al since the Ga is ontop of it (haha!) apply some stress. have fun. Just
don't do anything news worthy... and ruin this for the rest of us.
|
|
|
Craig
Harmless

Posts: 8
Registered: 4-5-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | | As BromicAcid said, I'd suggest you make some HgCl2 |
This is something I plan to do in the future.
| Quote: | | Some HCl might help to remove it |
I ordered some replacement HCl almost 2 weeks ago and I'm still waiting for it to arrive  . .
| Quote: | | If worse comes to worse I could send you some samples from my aluminum pile. |
Thanks for the offer  , but it's probably more trouble than it's worth.
If I harass enough aluminium companies I'm sure I'll get some samples in the end. , but it's probably more trouble than it's worth.
If I harass enough aluminium companies I'm sure I'll get some samples in the end.
To be honest, there were some parts of the process that didn't really make sense, but Uber Luminal's answer has made things clearer.
I'll probably use some of what you've said on my webpage  . .
|
|
|