| Pages:
1
..
27
28
29
30
31
..
81 |
greenlight
National Hazard
   
Posts: 753
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
You're right, an ammonium nitrate-based explosive would be better to break up rocks.
If you only had flash powder and no access to high explosives, it would work, but would be inefficient as you would have to use a lot more and stem
the boreholes etc.
But yeah, high explosives would be much better to use by a mile.
Be good, otherwise be good at it 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
One mole of H-C#C-H takes roughly 22.41 L and weights +/- 26g.
CaC2 + H2O --> CaO + H-C#C-H
CaC2 + 2 H2O --> Ca(OH)2 + H-C#C-H
Your silver acetylide nitrate double salt requires much less than 10g you need much less than 1 mole of CaC2/2,6...
----------------------------------------------------
Flash powder depending on its composition produces some gases; but if no gas is formed, then the gas trapped between the particles may expand
violently owing to the heat of reaction...
Pirat bangers containing flash powder are void for 4/5; only 1/5 is the flash; the rest is air entrapped in a solid container.
----------------------------------------------------
2 KNO3 + 4 Al + S → K2S + N2 + 2 Al2O3
doesn't take in account the formation of SO2 gas!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
The flash powder mixtures with Sulfur or black Antimony sulfide do have both temporary and permanent gasses from their reactions. They are also more
sensitive to work with- When young and foolish, some of us split up quite large, tough stumps with 50:25:25 Potassium chlorate:Antimony
sulfide:American dark Aluminum powder. Wouldn't make that mix again...
Ammonium perchlorate:Magnesium flash has virtually all gasseous output, some temporary, some permanent. If you must try this, coat the Mg powder with
dichromate using a Potassium dichromate solution and dry before mixing- And don't try to store it very long even then. See Shimizu FAST for details.
A (possibly safer) way to get some more gas output is to mix 50:50 flash powder:double based fast burning pistol or shotgun powder. Using equal
weights of the standard 70:30 perchlorate:Al flash and a fast, high NG content pistol powder such as Alliant "Bullseye" produces quite a lot of gas
& heat, hopefully without the added sensitivity of Sulfur or sulfides. I have NOT done formal drop hammer and friction testing on this mix, so
please don't make any assumptions as to it being particularly safe to handle-
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
ganger631
Hazard to Self
 
Posts: 53
Registered: 15-8-2014
Member Is Offline
Mood: Plata o plomo
|
|
Question regarding Silver acetylide, what is the difference between Silver acetylide double salt and just pure silver acetylide? As far as i know, it
just require extra nitric acid for its production and has higher vod. Are there any differences in terms of, stability, power and such.
|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  | Google IS your friend...
Calcium carbide reaction with water:
CaC2 + 2 H2O → C2H2 + Ca(OH)2
So a 1:1 ratio of carbide to gas. But your starting material may not be very pure. Carbide lamp fuel pellets, "Big Bang" cannon ammo, etc. certainly
are not.
Assume only 80% purity for such technical grade material, if freshly opened- Then do the stoichiometry. Need help with stoichiometry? We can move this
to beginnings.
|
Well, I thank you for the help, and I'm sorry if my question was vague, but that isn't actually what I meant.
You see, you have to bubble the acetylene through the solution of silver nitrate and nitric acid. I don't quite know how much of the acetylene in
these bubbles fully reacts before the bubbles escape form the solution. I don't think it all reacts does it? If that is the case, (and maybe I'm just
really really showing my ignorance here), then don't I need a good excess of gas, and thus more calcium carbide than stoichiometric quantity?
None of the synths I have found specified an amount, mostly mentioning the amount of time they bubbled acetylene, such as "till it looked like there
was no more precipitate forming" and "for about eight to ten minutes" and such. Such statements are rather unhelpful and seem to be unsound
approximations.
[Edited on 6-12-2015 by KesterDraconis]
|
|
|
Keith Fletcher
Harmless

Posts: 29
Registered: 3-10-2014
Location: Eastern US
Member Is Offline
Mood: Brittle
|
|
Hydrazine Hydrate Ethanol azeotrope
Dose Hydrazine Hydrate form an azeotrope with Anhydrous Ethanol and if so what is the boiling point?
My rule of life prescribed as an absolutely sacred rite smoking cigars and also the drinking of alcohol before, after and if need be during all meals
and in the intervals between them.
- Winston Churchill
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
While the dinitramide anion and its salts are well known, does anyone know of organic derivatives bearing that functional unit, i.e.
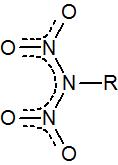
Can primary amines be doubly nitrated under special conditions?
[Edited on 6-12-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Nevermind, I found my answer to my own question in the first-page preview of "An Overview on the Synthetic Routes and Properties of Ammonium
Dinitramide (ADN) and other Dinitramide Salts" (requested).

|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ganger631  | | Question regarding Silver acetylide, what is the difference between Silver acetylide double salt and just pure silver acetylide? As far as i know, it
just require extra nitric acid for its production and has higher vod. Are there any differences in terms of, stability, power and such.
|
Silver acetylide Ag-C#C-Ag is formed from silver formate or acetate solution while bubbling acetylen through it.
As you can see it doesn't contain an oxydizer and so:
Ag-C#C-Ag --> 2 Ag + 2C
Only if air is present to some extend the following reaction occurs:
2C + 2 O2 --> 2 CO2
The double salt is Ag-C#C-Ag. x AgNO3. y HNO3 and it doesn't require nitric acid, only silver nitrate...
AgNO3 + H-C#C-H --> Ag-C#C-Ag. x AgNO3. y HNO3
As you can see there is nitrate oxydiser trapped inside the matrix. As such it produces of course more power and a higher VOD without need of external
oxygen.
The basic salt (Ag-C#C-Ag) is said to be more sensitive vs the neutral (Ag-C#C-Ag.x AgNO3) and acidic (Ag-C#C-Ag. x AgNO3. y HNO3) ones.
For stability, as long as they are done correctly (demi water, demi water washed paper filter, no sunlight or strong light, clean chemically pure
solvents, dried in the shadow at a moderate heat) and treated wel, they can store for years.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by KesterDraconis  | Quote: Originally posted by Bert  | Google IS your friend...
Calcium carbide reaction with water:
CaC2 + 2 H2O → C2H2 + Ca(OH)2
So a 1:1 ratio of carbide to gas. But your starting material may not be very pure. Carbide lamp fuel pellets, "Big Bang" cannon ammo, etc. certainly
are not.
Assume only 80% purity for such technical grade material, if freshly opened- Then do the stoichiometry. Need help with stoichiometry? We can move this
to beginnings.
|
Well, I thank you for the help, and I'm sorry if my question was vague, but that isn't actually what I meant.
You see, you have to bubble the acetylene through the solution of silver nitrate and nitric acid. I don't quite know how much of the acetylene in
these bubbles fully reacts before the bubbles escape form the solution. I don't think it all reacts does it? If that is the case, (and maybe I'm just
really really showing my ignorance here), then don't I need a good excess of gas, and thus more calcium carbide than stoichiometric quantity?
None of the synths I have found specified an amount, mostly mentioning the amount of time they bubbled acetylene, such as "till it looked like there
was no more precipitate forming" and "for about eight to ten minutes" and such. Such statements are rather unhelpful and seem to be unsound
approximations.
[Edited on 6-12-2015 by KesterDraconis] |
Usually the expensive material is the AgNO3 and not the acetylen from CaC2...thus as such one usually exhaust the AgNO3 solution with an excess of
acetylen by several filtrations, bubbling and demi water washings.
The white trouble is a clear evidence that some AgNO3 is stil present when bubbling acetylen through the solution...so filtration and washing then
rebubbling, refiltration and rewashing is the best way to improve yield.
This explains why it seems so unclear...also if you have an excess of AgNO3 vs the acetylen, you may get the not suitable complex Ag-C#C-Ag. 6 AgNO3
(not detonable) so better use an excess of acetylen.
Finally the best design to use acetylen at best is long vertical reactor with a slow flow of acetylen from the bottom (long plastic tube to the bottom
of a big vial for example); that way the gas has the time to dissolve a bit into the solution and to react with the AgNO3 all its way up to the
surface.
Acetylen is moderately soluble into water and the solubility is reduced by solutants (dissolved salts, acids and bases) (see solubility of gases in
water) and by heat.
--> Cold solution, avoid too much HNO3 (or avoid it at all), maybe add a little aceton (will boost up acetylen solubility).
[Edited on 10-12-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by deltaH  | | Nevermind, I found my answer to my own question in the first-page preview of "An Overview on the Synthetic Routes and Properties of Ammonium
Dinitramide (ADN) and other Dinitramide Salts" (requested). |
Organic dinitramides R-N(NO2)2 are relatively unstable.
You can check some related documents and infos in the chloronitramide (R-NCl-NO2) or ethylene dinitramide treads by Axt on this forum.
Beware that for EDNA (ethylene dinitramide) and MEDINA (methylene dinitramide) "dinitramide" refers to two nitramide groups -NH-NO2 and not to
dinitramide -N(NO2)2...thus not to confuse with possible
-methylene bis-dinitramide (CH2(-N(NO2)2)2 = CH2N6O8)
-ethylene bis-dinitramide ((O2N)2N-CH2-CH2-N(NO2)2 = C2H4N6O8)
[Edited on 11-12-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
Usually the expensive material is the AgNO3 and not the acetylen from CaC2...thus as such one usually exhaust the AgNO3 solution with an excess of
acetylen by several filtrations, bubbling and demi water washings.
The white trouble is a clear evidence that some AgNO3 is stil present when bubbling acetylen through the solution...so filtration and washing then
rebubbling, refiltration and rewashing is the best way to improve yield.
This explains why it seems so unclear...also if you have an excess of AgNO3 vs the acetylen, you may get the not suitable complex Ag-C#C-Ag. 6 AgNO3
(not detonable) so better use an excess of acetylen.
Finally the best design to use acetylen at best is long vertical reactor with a slow flow of acetylen from the bottom (long plastic tube to the bottom
of a big vial for example); that way the gas has the time to dissolve a bit into the solution and to react with the AgNO3 all its way up to the
surface.
Acetylen is moderately soluble into water and the solubility is reduced by solutants (dissolved salts, acids and bases) (see solubility of gases in
water) and by heat.
--> Cold solution, avoid too much HNO3 (or avoid it at all), maybe add a little aceton (will boost up acetylen solubility).
[Edited on 10-12-2015 by PHILOU Zrealone] |
Thanks, I suppose there really is no definite way to find smallest amount of acetylene necessary. Its ok for me though, I solved that problem
yesterday by finding a cheaper source of carbide, so I don't have to worry about saving it/running out nearly as much.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by KesterDraconis  | Quote: Originally posted by PHILOU Zrealone  |
Usually the expensive material is the AgNO3 and not the acetylen from CaC2...thus as such one usually exhaust the AgNO3 solution with an excess of
acetylen by several filtrations, bubbling and demi water washings.
The white trouble is a clear evidence that some AgNO3 is stil present when bubbling acetylen through the solution...so filtration and washing then
rebubbling, refiltration and rewashing is the best way to improve yield.
This explains why it seems so unclear...also if you have an excess of AgNO3 vs the acetylen, you may get the not suitable complex Ag-C#C-Ag. 6 AgNO3
(not detonable) so better use an excess of acetylen.
Finally the best design to use acetylen at best is long vertical reactor with a slow flow of acetylen from the bottom (long plastic tube to the bottom
of a big vial for example); that way the gas has the time to dissolve a bit into the solution and to react with the AgNO3 all its way up to the
surface.
Acetylen is moderately soluble into water and the solubility is reduced by solutants (dissolved salts, acids and bases) (see solubility of gases in
water) and by heat.
--> Cold solution, avoid too much HNO3 (or avoid it at all), maybe add a little aceton (will boost up acetylen solubility).
[Edited on 10-12-2015 by PHILOU Zrealone] |
Thanks, I suppose there really is no definite way to find smallest amount of acetylene necessary. Its ok for me though, I solved that problem
yesterday by finding a cheaper source of carbide, so I don't have to worry about saving it/running out nearly as much. |
There is, you simply need a closed reactor and a pressure gauge (manometer) or water displacement system and a recirculating device for the
undissolved/unreacted acetylen...while it reacts the initial volume will be reduced and the gaseous volume above the liquid will shrink...then by
trial error, you may find the minimal amount...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
There is, you simply need a closed reactor and a pressure gauge (manometer) or water displacement system and a recirculating device for the
undissolved/unreacted acetylen...while it reacts the initial volume will be reduced and the gaseous volume above the liquid will shrink...then by
trial error, you may find the minimal amount... |
Hmm, you know that sounds like it would be quite a bit of work or money to either construct or buy such a device. At the same time it sounds like
quite a bit of fun, and I think the information perhaps would be useful to people doing this synth in the future. I will consider it.
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
Sorry for interrupting this discussion 
Does someone have a paper about a one pot synthesis of carbohydrazide (diaminourea)
I already have a paper but with a two pot synthesis of carbohydrazide.
The problem is that it is designed for industrial use and not very commercial for me. 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Mr.Greeenix  | Sorry for interrupting this discussion 
Does someone have a paper about a one pot synthesis of carbohydrazide (diaminourea)
I already have a paper but with a two pot synthesis of carbohydrazide.
The problem is that it is designed for industrial use and not very commercial for me.  |
No paper but in principle reacting urea with concentrated hydrazine will evolve gaseous NH3 and leave you with aminourea and diaminourea (DAU)...
You may also work with common chemistry...
Phosgene + hydrazine -->DAU
Dimethyl carbonate + hydrazine --> DAU
...
[Edited on 16-12-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I found a product in a store with 40% Ethylene glycol.
I am not sure about the rest 60% of the product. I am not sure if it is water or antirust material.
is it possible to get concentrated Ethylene glycol from this product. I was thinking about distillation but glycol has boiling point of 190 degrees
and if I have water in the solution this won't work as I think.
any suggestion ?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
From what I have seen the anti corrosion additive is normally in very small quantity. It depends on what the climate is like where you are how much,
and if, antifreeze needs to be used at all; in Las Vegas I read that anti corrosion chemicals are added to plain deionized water and that can be used
as coolant, where I am it gets pretty cold so 50% ethylene glycol is normally used (considerably less concentration could normally be used, but 50%
gives a good margin of safety for really low temperatures and possible reduction in ethylene glycol concentration over time, etc). I can get 50%
concentration which is made to be poured right into the rad without dilution and basically 100% concentration which is meant to be diluted with
distilled or deionized water.
It has been a few years since I have distilled any but from what I remember it is extremely easy to distill with very high purity, concentration and
recovery with even a very simple pot still and condenser (one stage). The still needs to be able to handle 200C or more and ethylene glycol dissolves
most normal rubbers and plastics from what I have seen (ruined a few items playing with it originally), but something all metal can be easily
improvised. I have only ever distilled the undiluted antifreeze, but the 50% or 40% variety could be distilled too if none of the 100% stuff could be
found. When distilling 1L or so of the "100%" variety I remember a couple millilitres or so of nearly pure water coming over at around 100C and then
literally only a couple drops coming over as the temperature rose from 100C to about 190C. At around 190-200C or so a steady stream of practically
pure ethylene glycol came over. I remember getting significantly better yields of EGDN using the distilled ethylene glycol than NG when using
glycerine from the drug store using the same acids.
[Edited on 29-12-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
you can by almost pure ethylene glycol if you get concentrated coolant.
Beginning construction of periodic table display
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
@Hennig,
If 60% is distilled water , I think water will evaporate at 100C before Glycol. do I need to add H2SO4 to avoid water evaporation ?
I also can heat till 100 C till water get out then start to distill and collect the concentrated Glycol.
If 60% is antirust, what should I do now ? 
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
The coolant I had was a florescent green type color IIRC. I distilled to remove the tiny amount of water it contained but mostly to separate it from
the dye and other higher boiling point materials. The distillate which came over at around 190-200C was crystal clear and basically anhydrous.
Ok, found some equilibrium data, there will be some losses especially when starting with coolant with a high concentration of water.
The equilibrium data came by way of the Wiki ethylene glycol data page. The data actually came from the following chemical engineering research
database however.
http://www.cheric.org/research/kdb/hcvle/showvle.php?vleid=1...
I added a couple of columns and made the table into a pdf. Hope I didn't mess up the calculations, I feel rusty.
Attachment: Ethylene Glycol-Water Equilibrium Data.pdf (296kB)
This file has been downloaded 642 times
Here is an ethylene glycol data page as well.
Attachment: Ethylene Glycol Data Page.pdf (1.9MB)
This file has been downloaded 632 times
[Edited on 29-12-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
I preformed a similar distillation of the glycol from the dark green dye and was very pleased with the very small amount of water that had to be
disrcarded. ~35ml
I just began collecting around 180C.
The type I used was given to my by elementcollector and came from a black jug. It was meant to be used as a an antifreeze for RVs IIRC.
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
I have been told not to install an electrical outlet inside of my fume hood, I assume because of the risk of explosion. Is it really that unsafe? I
hoped that by placing it near the sash I could minimise this chance greatly, it certainly seems safer than stringing cords around he outside...
Any input would be greatly appreciated.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
It depends on what you are doing in your fume hood, but NOT having any non explosion proof electricals inside your hood gives you greater flexibility
in what you CAN do safely.
Electric sparks and flammable/explosive vapors and dusts are a bad mix, M'kay? I have heard first hand horror stories from people about using diethyl
ether and throwing a standard wall light switch, just before they lost their lab. How they saw that little flame exit the switch plate, right before
the open solvent filled container exploded.
Alternatively, you can obtain explosion proof electrical boxes, use heavy walled conduit and explosive atmosphere rated wiring devices and feeds. We
have done this in one shipping warehouse/magazine that needs to be used any time of day, the equipment is VERY expensive.
We also have a small prefab building just for handling explosives in preparation and re-packing. There are no electrical circuits inside it- Exterior
LED lights shining in through windows near the roof allow use after dark. All conduits, electrical boxes and switches are OUTSIDE the building.
Cheapest reasonably safe solution.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
idrbur
Hazard to Self
 
Posts: 88
Registered: 23-6-2015
Member Is Offline
Mood: No Mood
|
|
picric acid
Somedays before i tried to make PA with aspirin but i obtained only few crystals .so i tried again but this time i doesn't get precipitate so i cold
the solution to -3 degree but it doesn't worked .so again performed the process with new batches 2-3 times but i doesn't get ppt. Any time .
Can anybody tell me how can i precipitate picric acid from the solution.
|
|
|
Texium
|
Threads Merged
31-12-2015 at 07:24 |
Texium
|
Thread Topped
31-12-2015 at 07:24 |
| Pages:
1
..
27
28
29
30
31
..
81 |