froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
Iron-Chlorine secondary cell - conceptually?
I've had this idea for an Iron Chlorine rechargeable cell for a while now and would like to run it by the folks here to get an idea on it's
feasibility.
I have attached a basic diagram of the cell.
The intended half reactions for this cell are:
Discharge:
Anode: Fe -> Fe^3+ + 3e^- (+0.04V)
Cathode: 2Cl^- -> Cl2(g) + 2e^- (+1.36V)
Cell Voltage is 1,4V
(ref: https://en.wikipedia.org/wiki/Standard_electrode_potential_%...)
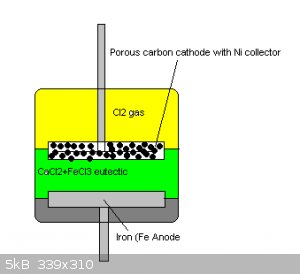
This cell employs an anhydrous deep eutectic combination of Ferric chloride and Calcium chloride whereby the excess Ferric chloride formed during
discharge would remain in and form part of the electrolyte.
(referring to my post here: https://www.sciencemadness.org/whisper/viewthread.php?tid=10...)
The Cathode is a porous carbon electrode with a nickel mesh current collector hopefully in order to effectively collect and diffuse chlorine gas
during cycling.
The cell/cell stack would be constructed inside a chlorine resistant pressure vessel that would store the chlorine gas produced after charging under
pressure.
According to the phase diagram this cell should operate well within it's parameters at around 30 degC provided the molar proportion is maintained.
I know there are people here with way more electrochemical experience than I have and would like to hear their views on the concept along with any
advice or suggestions.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I would consider the volume x pressure of chlorine gas required for your required A.h capacity
Then consider that quantity in a consumer environment.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hey froot, nice to see a thread by you and an idea for an invention to boot, keep them coming!
Now down to the business. Whenever you have gas and phase change as one of your half reactions, chances are that you will need a catalyst to get
decent amounts of power out of the system. For chlorine, this is quite easy, it so happens that ruthenium dioxide is a fantastic chlorine catalyst, so
DO use RuO2 MMO titanium anodes. In SA there's a great company that makes them, NMT electrodes. They are also even cheaper than China!
Secondly, your cell voltage might not be 1.4V because that's defined for a 1M Cl- solution as electrolyte in water, but it would probably be close to
that anyhow.
Next, I've experimented with DES electrolytes for batteries and found their performance wanting, sadly! Specifically, they have much lower
conductivity compared to traditional good electrolytes like concentrated KOH or H2SO4 solutions in water. The mobility of the ions seems to be low.
Again, it'll give a voltage, but don't expect power densities anywhere near conventional batteries.
Running hot on the other hand helps A LOT in this regard. But then you might as well run a hot molten salt battery.
[Edited on 23-11-2015 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Two problems I see.
One is that the eutectic needs to be maintained to within certain specifications -- which means that the amount of FeCl3 used or liberated must be
kept relatively small. This means that it is not going to be a deep-cycling battery.
Secondly, it does not look like an entirely user-friendly idea. Free chlorine in a consumer product is something generally to avoid.
I'll let the experts chime in in the chemistry of it all.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
There are better ways to skin a mule.
http://www.google.com/patents/US5422197
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Feel free to balance this equation
Fe + FeCl3 --> FeCl2
then explain what will stop it happening in the cell.
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
Unionised that was one of my concerns and suspected this would be a favorable reaction in those conditions but this got me thinking - baseless
speculation as follows:
Anhydrous FeCl2 is an amorphous solid it may be possible that it forms a 'passivation' layer on the iron anode preventing further reaction with FeCl3
in anhydrous conditions. Now would this inhibit electrochemical functionality of the cell or could it improve it?
Half reactions from wiki I mentioned above:
1) Fe^3+ + e^− -> Fe^2+ +0.77V
2) Fe^2+ + 2^ e− -> Fe(s) −0.44V
If my 'passivation' theory is correct and equation 1 occurs then this is proof that Cl^- ion mobility occurs. How the +0,77V, being on the electrolyte
side of the 'passivation' layer affects the bigger picture and if it determines the favourability of this reaction is a bit beyond my understanding
and am hoping for some input on this. Furthermore equation 2 would suggest an increased overall cell voltage.
If I'm making a fool of myself it's worth the 1 in 1000 chance that I'm onto something.
j_sum1 If you look at the phase diagram I may be reading it wrong but as the FeCl3 molarity increases it remains liquid but with solid FeCl3 suspended
in it so one may be able to achieve an acceptable cycle depth from it.
As for the chlorine hazard I would encase this cell chamber in a second low pressure chamber with an inch of ammonia solution at the bottom which
would neutralise any chlorine that happened to leak from the cell chamber.
DeltaH I'd be interested in your experiments and findings from working with DES electrolytes. I've always speculated that anhydrous electrolytes would
perform far better than aqueous electrolytes especially from a charge efficiency and ion vs electron conductivity perspective. Thanks for the info on
the chlorine electrode.
Thanks for the input.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I have tried the choline chloride + metal chloride 'flavour', not anhydrous (75% choline chloride solution was used), nevertheless, conductivity was
dramatically poorer compared to acid in water and KOH in water.
It may be mixture specific, but I think the trend will hold. Few things come close to the ease with which protons hop between water molecules.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Why is that so ?
Kinetics ?
If so, the notion of how it all works may be influenced by something as simple as agitation of the electrolyte.
A purely chemical system might not work, wheras a hybrid might.
Edit:
Odd feeling.
2 years in and i'm suggesting mechanical vibration as a catalyst in an electrochemical reaction.
Very odd indeed.
[Edited on 25-11-2015 by aga]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yes it's a kinetic effect born out of a special mechanism of ionic flow, called the Grotthuss mechanism and you can read a nice introduction about it
here and also see a nifty animation:
https://en.wikipedia.org/wiki/Grotthuss_mechanism
From the article:
| Quote: | | The Grotthuss mechanism, along with the relative lightness and small size of the proton, explains the unusually high diffusion rate of the proton
relative to that of other common cations (Table 1), which is due simply to random thermal motion, i.e. Brownian motion. Quantum tunnelling becomes
more probable the smaller the mass of the cation is, and the proton is the lightest possible stable cation. Thus there is a minor effect from quantum
tunnelling also, although it dominates at low temperatures only. |
Also from the article:
Table 1 values:
K+ 0.762×10−3 Mobility / cm2 V−1 s−1
H+ 3.62×10−3 Mobility / cm2 V−1 s−1
To put that into perspective (provided I am interpreting that table correctly) if you used potassium sulfate as electrolyte in your car battery
instead of sulfuric acid of the same molarity, instead of bursting out say 340A during starting, it would produce a feeble ~72A initially, 4.75 times
less!
What's more, those ionic mobilities are for solutions in water...
I know that for the electrolyte of lithium ion batteries, it's common to use a solvent combination of ethylene carbonate and dimethoxy methane, as an
anhydrous solvent for lithium ions, specifically.
Nafion membranes, being a polymer of a super-acid, has a very high proton conductivity, but it's price is insane!
[Edited on 26-11-2015 by deltaH]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Either an alternative or pricing will come down on the nafion membranes if flow cell technology will come online. Distributed power storage, everyone
needs one in the garage.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I have seen cheaper hybrid anionic membranes sold commercially at much lower prices than Nafion, however, on closer inspection, their conductivity is
rather pathetic and those with high conductivity cost nearly as much as Nafion 
[Edited on 26-11-2015 by deltaH]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Would surely be nice to be able to manufacture your own membranes, but I'm probably dreaming there. Anyone have any idea whats involved?
Maybe it's not so far fetched. Can anyone access this article? Looks interesting.
Now we just need a supply of rejects.
http://www.sciencedirect.com/science/article/pii/S0013468604...
And another one of interest.
http://www.sciencedirect.com/science/article/pii/S0167273898...
[Edited on 26-11-2015 by hyfalcon]
[Edited on 26-11-2015 by hyfalcon]
[Edited on 26-11-2015 by hyfalcon]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The hybrid types are potentially possible in the amateur context, but the downside is they're not very conductive, I think something in the order of
300A/m^2 of membrane (the pro ones), which is pretty poor.
I haven't looked into it, but my understanding is that you start from ordinary cheap ion exchange resin, mill it, then mix with an inert polymer ?like
LDPE? and then extrude as thin a sheet as possible.
A very crude version might be hacked in an amateur context, press a melt from glue-gun sticks and ionic resin in a smooth vice with waxed paper (used
in baking) squeezed between two hot metal plates?
|
|
|
Geekineer
Harmless

Posts: 12
Registered: 21-12-2015
Member Is Offline
Mood: No Mood
|
|
3D printing ionic membranes
What about taking the milled ionic resins and extruding it into 1.75mm filament for 3D printing - not sure if you'd have to mix in some PLA ...... but
there are a lot of additives out there today and why not try one more.
|
|
|