Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
boiling in a tube
If there was a VOC, such a petroleum ether placed into a tube that was closed on one end and heated, how far would the vapors travel up the tube
before condensing?
I think as the vapor and heat moves across the glass it would become heated, making the vapor keep traveling. I hypothesize that the vapor would fill
the tube, and the bottom of the tube would be dry.
How far would the vapor go in a tube? I'm wondering if vocs can be refluxed without a water cooled condenser, and if so how would it be determined the
length of tubing needed for this to occur?
is this not an enthalpy problem? or a conduction type problem? How can some things condense without water coooling? wouldnt the vapor bring heat up
through the condenser, along with heat radiating through the glass , that would keep the vapor a vapor and prevent condensation?
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
You'd need a condenser. Eventually, after a good bit of boiling, the glass would heat up enough not to condense all of the solvent; it would start to
escape out the top. Things only condense without water cooling if they've got a cool surface to condense on (or pressure).
The idea of a condenser is basically to carry heat away from the vapor and put it somewhere else. Say you hook up your condenser to a tap and let it
drain into the sink. The condenser is picking heat up from the vapor inside, allowing it to condense. The heated water then leaves the condenser and
goes down the drain, effectively "stealing" the heat from the solvent. We use water in condensers because it's common (duh) and it's got a high
specific heat capacity, so the flow can be relatively slow.
That's the concept of "heat of vaporization." The condenser must be carrying energy away as fast as the heat source is pumping it in. For compounds
with greater Delta Hvap, more energy is required to boil the solvent, but it releases more energy in the condenser when the change is
reversed.
Fear is what you get when caution wasn't enough.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Another question, what happens when a pressure cooker reaches its pressure but is still on the heat? according to the tripple point diagram for water,
at 15psi (roughly 1 atmosphere) and at around 100 degrees centigrade water is a vapor.
does this pressure prevent more water from boiling? At a certain temperature and pressure such as around 1 atmosphere and 100degrees c in the diagram
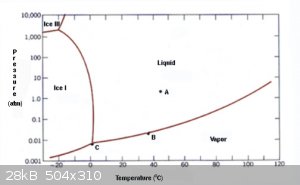
what happens on the red line? is there where no more water will turn to vapor, the pressure wont rise, so long as the temperature is kept at 100 c?
what happen inside the cooker? is the vapor and liquid at equilibrium or something? or the rates at which the phases change from gas to liquid at
equilibrium?
[Edited on 11/17/2015 by Yttrium2]
|
|
|
Deathunter88
National Hazard
   
Posts: 522
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
An air condenser is used for substances boiling higher than 150 degrees C.
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Yttrium2  | Another question, what happens when a pressure cooker reaches its pressure but is still on the heat? according to the tripple point diagram for water,
at 15psi (roughly 1 atmosphere) and at around 100 degrees centigrade water is a vapor.
does this pressure prevent more water from boiling? At a certain temperature and pressure such as around 1 atmosphere and 100degrees c in the diagram
http://postimage.org/
what happens on the red line? is there where no more water will turn to vapor, the pressure wont rise, so long as the temperature is kept at 100 c?
what happen inside the cooker? is the vapor and liquid at equilibrium or something? or the rates at which the phases change from gas to liquid at
equilibrium?
[Edited on 11/17/2015 by Yttrium2] |
You are asking 2 questions here. As to what happens when a pressure cooker reaches "its pressure", there is a pressure relief valve which releases
steam until the pressure returns to the chosen level. You don't have control over this level, it is a safety feature.
As to the operation of a pressure cooker, as you correctly understood from your diagram, water heated to 100 degC at 1 atm turns to vapor and normally
leaves the heating vessel, So under these conditions, water can never be heated above 100 degC. No matter how strong your fire is, it will just make
more water vapor which will carry off enough heat energy to keep the remaining water at 100 degC.
Pressure cookers seal the vapor in, so it can't escape. Thus the pressure can rise to higher than one atm. How much pressure is determined by how high
the temperature of the water gets, and as your chart shows, water can remain liquid at temps higher than 100 degC if it is under pressure higher than
1 atm. So, a part of the water is turned to vapor, but the remainder of it stays as super-hot liquid. Because both phases of water are present, the
water will follow the line on your chart that is between the vapor area and the liquid area, i.e., the line that point B is sitting on.
Therefore, the usefulness of a pressure cooker for cooking food is to allow temps greater than 100 degC, which allows your food to cook faster, or to
better sterilize utensils, canning jars, etc.
[Edited on 18-11-2015 by Artemus Gordon]
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Quote: Originally posted by Artemus Gordon  | Quote: Originally posted by Yttrium2  | Another question, what happens when a pressure cooker reaches its pressure but is still on the heat? according to the tripple point diagram for water,
at 15psi (roughly 1 atmosphere) and at around 100 degrees centigrade water is a vapor.
does this pressure prevent more water from boiling? At a certain temperature and pressure such as around 1 atmosphere and 100degrees c in the diagram
http://postimage.org/
what happens on the red line? is there where no more water will turn to vapor, the pressure wont rise, so long as the temperature is kept at 100 c?
what happen inside the cooker? is the vapor and liquid at equilibrium or something? or the rates at which the phases change from gas to liquid at
equilibrium?
[Edited on 11/17/2015 by Yttrium2] |
You are asking 2 questions here. As to what happens when a pressure cooker reaches "its pressure", there is a pressure relief valve which releases
steam until the pressure returns to the chosen level. You don't have control over this level, it is a safety feature.
As to the operation of a pressure cooker, as you correctly understood from your diagram, water heated to 100 degC at 1 atm turns to vapor and normally
leaves the heating vessel, So under these conditions, water can never be heated above 100 degC. No matter how strong your fire is, it will just make
more water vapor which will carry off enough heat energy to keep the remaining water at 100 degC.
Pressure cookers seal the vapor in, so it can't escape. Thus the pressure can rise to higher than one atm. How much pressure is determined by how high
the temperature of the water gets, and as your chart shows, water can remain liquid at temps higher than 100 degC if it is under pressure higher than
1 atm. So, a part of the water is turned to vapor, but the remainder of it stays as super-hot liquid. Because both phases of water are present, the
water will follow the line on your chart that is between the vapor area and the liquid area, i.e., the line that point B is sitting on.
Therefore, the usefulness of a pressure cooker for cooking food is to allow temps greater than 100 degC, which allows your food to cook faster, or to
better sterilize utensils, canning jars, etc.
[Edited on 18-11-2015 by Artemus Gordon] |
I guess I was not clear on my question. I'm wondering what happens if the temperature doesnt change. If it stays at 100C and if the pressure stays at
1 atmosphere.
What happens then? What is it called when it is on the line between vapor and liquid? No additional pressure is created without a change of
temperature, because of this, I dont believe it creates more and more water vapor. If more and more vapor was created at constant temperature and
pressure the pressure would rise, this doesnt make sense even, does it?
the term is critical point, where water and vapor are in equilibrium, whatever that means.. Does this means half the molecules will be gas and half
will be liquid? I dont think so? I could be wrong, I think it means that half will be condensing whereas the other half will be evaporating??? - I
guess thats really the same as half being liquid and half being vapor.. So what I guess... ? lol
(why is this useful?)
[Edited on 11/18/2015 by Yttrium2]
[Edited on 11/18/2015 by Yttrium2]
[Edited on 11/18/2015 by Yttrium2]
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Is there anyways to determine how long an aircooled condenser would need to be without trying it out?
What factors would need to be known? For example, volume of gas?
[Edited on 11/20/2015 by Yttrium2]
|
|
|
Fulmen
International Hazard
    
Posts: 1726
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Not really. When you heat a liquid the vapor pressure increases. At some point (100°C for water) the vapor pressure reaches 1 atmosphere, so at
normal pressures it begins to boil. Keep pumping energy into the liquid and you'll boil it away, the temperature stays at 100°C because the ambient
pressure is 1atm.
A pressure cooker is a pressure vessel, so no vapor can escape at first. So what happens as the liquid starts to boil? The vapors build up, causing an
increase in pressure. When this happens the water stops boiling since the pressure is now higher than the vapor pressure. So the energy you add goes
into heating the liquid further, raising the vapor pressure until it once again equals the pressure inside the cooker. But as soon as it boils, the
pressure increases again.
This process continues until the safety valve activates (at appr. 2 atm). At this point the liquid boils (at around 120°C), but now the valve
prevents the pressure from increasing any further so the liquid will boil steadily until it's gone.
As for air cooled condensers: Yes, it's possible to calculate but it's pretty challenging. You'll need to factor in the liquids boiling point, ambient
temperatures, power dissipation, external surface area, thermal conductivity, any forced air currents and condenser configuration in order to
calculate convection rates. And calculating heat convection requires a lot of math. So short answer: no.
[Edited on 20-11-15 by Fulmen]
We're not banging rocks together here. We know how to put a man back together.
|
|
|
|