Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
Analyzing Arsenic
hello all,
i recently bought a bottle of arsenic pills, originally from pharmacy ''Cretin'' that dates back about 100 years (atleast the bottle does) but i don't
know if the pills contained are actually arsenic. does anybody have an idea if i can see if they are actually arsenic? (preferably not testing it out
on a rat)
i will include a pic as soon as i find my camera.
thanks,
Alexander
all above information is intellectual property of Pyro.  |
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Your best way forward is a simple Marsh test.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
ok, could you please explain some more? that in unfamiliar to me. i'm looking it up now.
EDIT:i will have to do the one with carbon, as it isnt a good idea to smell AsH3
it sounds a bit scary, any1 got a bit of advice?
[Edited on 26-5-2012 by Pyro]
all above information is intellectual property of Pyro.  |
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
The sample is placed in a gas generator containing HCl, and some zinc is added. The nozzle of the gas generator should be drawn to a fine tip (not too
fine). The gas stream is lit and the flame passed under a white tile. If the sample contains antimony or arsenic, the flame will leave a streak. If
you put bleach on the streak, you can tell whether the sample had antimony or arsenic. If it's arsenic, the streak will disappear, but if it's
antimony, it will be unaffected.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Nothing will happen to you if you feel the smell of AsH3. It's not like you're making liters of it. It's an analytical technique. You won't die, you
won't get sick.
Use a test tube as a reaction vessel and a small tube like this one.
Use analytical grade zinc, and make a blind probe just to be sure the reagent doesn't have enough arsenic to be detectable by your setup.
Technical grade zinc gave weak positive results on arsenic in my setup, but as I wanted to make a really nice arsenic mirror, I've added few drops of
0.1 M sodium arsenate.
You've probably sniffed AsH3 before. It's one of the compounds that give that peculiar smell of onions when technical grade zinc is dissolved in
acids.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
so i put my suposed As2O3 in some HCl and put zinc in, then burn the gas under a tile, and then wipe with bleach.
am i correct? i suppose i need to be super careful of breathing the gas...
all above information is intellectual property of Pyro.  |
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Use minute quantities of the sample. Marsh probe is a very sensitive qualitative technique.
The method barley81 described is an "arsenic spot" test. I wouldn't recommend it to a beginner as it releases too much arsine in the air.
Just make a standard gas generator using a test tube, as if you're making an experiment for kids in the classroom showing the properties of hydrogen,
but instead of using a short burning tube, use a longer one, bent like the one I took photo of. It's advisable to put a calcium chloride tube before
the main tube, because you want just the arsine, and not hydrogen chloride, too.
After you check for hydrogen purity, light the flame at the tip of the heating tube. Depending on the arsenic content, more of less prominent blue
flame of burning arsine appears, giving off white fumes of arsenic trioxide. Of course, avoid breathing it. 
When you start heating one part of the main tube, arsine decomposes to arsenic and hydrogen. Arsenic mirror will form at the heated spot of the tube,
and the flame turns to a nearly invisible hydrogen flame.
Of course, if there's any sodium in the sample, and chances there is, the flame will always have a yellow color. It's an annoying nuisance.
Afterwards, you can do the bleach test, as barley81 said, to distinguish arsenic from antimony.
Arsenic pills, you say? Can you take a photo of the bottle and the contents?
[Edited on 26-5-2012 by Endimion17]
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Huh. I didn't know that. I found the procedure for the test in "Illustrated Guide to Home Chemistry Experiments" by Robert Bruce Thompson. Wikipedia's
description of the test is very similar.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
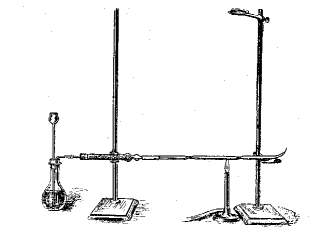
Forensic science is awesome. Too bad it's tough to get a job in that field.
I like these old experiments. The one with WP poisoning is cool, too, because it gives a luminous ring.
Today, it's all about equipment with buttons and dials. Granted, the tests are more sensitive, but less enchanting. 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Arsenic is is a Group II metal and can be identified by a classic wet chemical qualitative analysis scheme. But you would have to get it into the
As+++ or AsO4--- form, I believe.
You might try dissovling some of your pills in water then filtering. Treat the filtrate with H202 at high pH (use NaOH) to convert As+++ to AsO4---.
Then treat this with MgCl2/NH4Cl. A white ppt of MgNH4AsO4 will form if AsO4--- is present.
You should get a proper procedure off the internet if you decide to try this.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
as i said earlier, i have lost my camera.
it's a light brown bottle, with a wide top, it is 13 centimetres high, 5,2 cm across, the neck is 3 cm across and 3 cm long is full of little whitish
pills about 3mm across and has a small enlargement at the top, it has a cork stopper and a very old label saying:
pharmacie
H. CRETIN
8, rue des eubrons-Bruxelles
telephone: 33 47 60
then in handwriting:
Granules a l'arsenite
de scrude a limfer
and at the very top it says: 0,25 piece
and a round sticker with skull and crossbones saying: vergift-poison
thanks for all the advice, i'm going to look for my camera
its hard to read, but this is roughly what it means, pharmacy
the name, funny thing is that cretin in french means little bastard, and in english is a mentally retardid dwarf
the adress
the telephone number
arsenic granules
don't know what the next means,
at the very top i imagine is either the amount of arsenic in every pill, or the weight of every pill
EDIT: i got pics, sorry they are in word, i dont know any other way to post em
[Edited on 27-5-2012 by Pyro]
Attachment: pics of As pills.docx (439kB)
This file has been downloaded 396 times
all above information is intellectual property of Pyro.  |
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
So, what is the end of the story? Did you ever analyze the pills? What did you find out? I'm waiting with bated breath!
|
|
|