| Pages:
1
2 |
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
And even then there will probably be significant losses since you're starting out with perhaps 3-40000ppm at best. Of course a relatively large volume
shouldn't be too hard to achieve considering the size of a home setup, but ozone (perhaps in a secondary stage) has the potential to increase yields
and limit the need for exhaust gas scrubbers. And if one follows WGTRs design with HV ignition of a LV arc you already have the HV needed to generate
ozone.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
And if correctly configured, it would eliminate the need for absorption towers altogether!
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
It does seem like a realistic approach, but we need a lot more data on the N2O4/O3-reaction. This seems like a good starting point:
http://pubs.acs.org/doi/abs/10.1021/bk-1996-0623.ch008
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Is there any reason to suspect that passing NO/NO2 through an ozone generator could destroy any of the nitric oxides? Industrially they seem to
produce it separately, but that would likely be for process control. Assuming all NO is oxidized to N2O5 you would get a theoretical yield of 0,88g
HNO3 per gram O3, the numbers should be far better if the initial NO is allowed to react with air. Running the output from the arc reactor through an
initial absorption column (producing weak HNO3), then ozonating the remainder before absorbing it in concentrated acid should produce a quite
effective setup for FNA.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I've been following the posts in this thread with interest recently, but things have been busy during the past several days. I'm not yet able to
interject a post that will do complete justice to the contributions from the posters here.
I agree that ozone seems to be an attractive choice to promote full absorption of nitric gasses. However, I think it would have to be generated
inline to the NO2 stream, not added separately. This avoids the further dilution of the NO2 gas stream. On the other hand, NO
decomposes O3 catalytically in the presence of oxygen radicals.
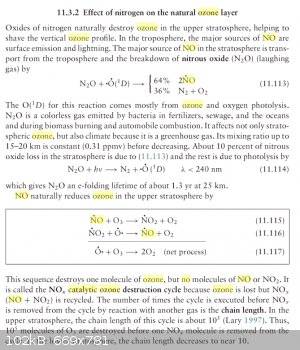
Fundamentals of Atmospheric Modeling, Mark Z. Jacobson
This would seem to suggest that perhaps NO needs to be kept out of the O3 generator.
In Webb's book, he stated that ozone generally affects a change to N2O5 with only about 33% efficiency.
I realize that there is a lot of interest in making fuming nitric acid, but I think that any system we make will be producing diluted streams of acid.
There are several reasons for this. For one, 20% acid can be attained from very dilute acid simply by boiling it down in a beaker. I'm working from
memory on this one, but I believe that after boiling out 1/2 of the acid (on a HNO3 basis) from a dilute solution, the residue is already
constant boiling 68% acid. In any case, it's not hard to concentrate it. Fuming nitric can be made the usual way, with concentrated sulfuric acid,
if someone wants to go through the effort. What we don't want to do is offer a commercial product that someone can directly use to make explosives.
That would be a bad idea, and bad press, in my estimation. The current EU regulations that limit commercial nitric acid to 3% is not so much a
problem in itself. The main problem is that the cost for the 3% acid will likely be very uneconomical, like buying 3% H2O2 at
the grocery store is. What would be interesting to know, is if the 3% acid is widely available in the EU now, and what its cost is. Making your
own 3% acid would save money over time, if significant quantities were being used. Also, N2O5 is somewhat unstable
according to Webb, and extra precautions would need to be taken if this was part of the process.
I'd like to scan Webb's book for the library here, if I can get the time soon. We already have a copy here, but several pages are illegible, and a significant number of important charts and illustrations are missing.
Anyway, enjoy!
Attachment: Oxidation_of_Nitric_Oxide_in_Two-Stage_Chemical_Scrubbers_Using_DC_Corona_Discharge.pdf (4.7MB)
This file has been downloaded 729 times
[Edited on 10-26-2015 by WGTR]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Wow, haven't had the time to read the entire JHM-article, but it seems to confirm our hypothesis.
For a commerical setup I agree that fuming acid isn't that interesting, and it would probably only be achievable with significant loss of yield. But
for a home setup I think it's at least worth exploring.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
antimon
Harmless

Posts: 33
Registered: 24-6-2015
Member Is Offline
Mood: No Mood
|
|
Hi, i have read the threads here on the forum about this reactor, and i must say that i have become a lot clearer about how i am going to construct
it.
I have planned to use an ignition coil all this time, but after researching it i decided togo with a NST. I ordered a 6kV neon sign transformer from
ebay yesterday. So i hope that will make things easier for my project.
I would like to see whats going on so i am going to use a large glass jar for the arc chamber. Now i am just waiting for some glass drill bits that i
ordered earlier this week.
And that´s what i am wondering about. I am a little bit unsure how many holes i will need to drill in the jar, so i wanted to ask you.
1. One inlet for the pump, right?
2. And then an outlet so that the NO can get out of there to the bubblers.
Are there any more?
Lastly, i am almost 100% sure that i am going with a stationary arc, instead of a ladder. So i am going to drill a hole on each side of the jar for
the electrodes to sit in.
I had a whole bunch of questions but i can´t come to think of any right now. I´ll be back later this weekend.
Thanks for all your posts. I appreciate it.
|
|
|
antimon
Harmless

Posts: 33
Registered: 24-6-2015
Member Is Offline
Mood: No Mood
|
|
Ps. Does anyone here have experience with electric arcs, and magnetism?
|
|
|
Aurium
Harmless

Posts: 46
Registered: 4-10-2015
Member Is Offline
Mood: Energetic
|
|
You need the high voltage to trigger an arc discharge to jump across the terminals, once the initial arc is formed, it acts as a very very low
resistor, very conductive, between the terminals.
This shorting of the terminals will allow a high current to flow, decreasing voltage to almost nothing and overloading the power supply, reducing
power transfer and efficiency, allot.
If the goal is to create the largest, hotter arcs, perhaps we can try using a power supply with lower impedance, like a microwave oven transformer
(MOT) or a welding power supply.
These sources don't have enough voltage to strike an arc, but when arc is formed, the higher currents delivered cause huge huge arcs.
To get an arc started one could touch the terminals together or superimpose a small HV power supply, just to trigger the arc.
This is exactly how many guys make homemade Arc Furnaces. 2 MOTs recoiled to give some 48V at 1Shitton of amps can keep a long arc.
Even a regular MOT at 2200V can make 30cm arcs.
WGTR is following this technique and I think he's right on the money here.
I'v been reading your older posts about the electrodes corroding away.
Have you tried using graphite rods from batteries?
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
antimon
Link to the part that you ordered?
Glass should be drilled while submerged under water. It's less likely to crack this way. Still, it will take considerable patience.
The number of holes sounds about right.
Compressed air is one option for an air supply. Also, a weighted trash bag full of air can be used, and this is similar to how old pipe organs used
weighted bellows to regulate air pressure.
The last reactor that I posted on here was built with clay. If my memory is correct, I used A.P. Green fireclay, grog, and about 10% OM-4 ball clay
for added plasticity. This was bisque fired at 1000C for a couple of hours (Bisque, not biscuit. What, do I look like Martha
Stewart?!?). This firing schedule wasn't intended for vitrification, only to achieve suitable strength for handling.
It's probably better to use a strong rare-earth magnet to try deflecting the arc first, until you learn how this works. Don't hold the magnet close
to the arc with your hands!!! Bzzzt!! Owww!! Owww!!!
|
|
|
antimon
Harmless

Posts: 33
Registered: 24-6-2015
Member Is Offline
Mood: No Mood
|
|
WGTR : The site is in swedish, so i will try to find one on eBay.
I ordered a Dremel 3000 and two different drill bits.
Here it is.
http://m.ebay.com/itm/Dremel-662-Glass-Drilling-Diamond-Dril...
Would you use a stationary arc, or a ladder? It seems like its easier to flatten it out if its stationary.
Thanks for the tip. I will use a stick or something. 
Aurium : I dont know if the strongest hottest arc is what you should aim for.
I am in the process of making a Jacobs ladder with one mot. But i am thinking of using one more in series.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I imagine that using an NST will make the electrodes fairly hot
and using an MOT will make them VERY hot
possibly hot enough to soften or even melt glass
so a pottery vessel and/or heatsinking for the electrodes are required I suspect.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
TIG electrodes should be the obvious choice, they're not expensive and readily available.
For the PSU I agree that WTGR probably has the simplest and best method. And as it happens I do have a complete ignition coil & driver lying, so I
just might have to test this one of these days. I also have an 8A variac that should be suitable for supplying power at varying voltages.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
antimon
Harmless

Posts: 33
Registered: 24-6-2015
Member Is Offline
Mood: No Mood
|
|
I recieved my 6 kV NST today, and when i opened the box and i checked the output and i saw that it said: 3.2kV-E-3.2kV 24mA 30 kHz.
Does this mean i have been cheated, or do the leads put out 3.2 kV each? Probably not i suppose.
But on the pro side it seems to have a knob that i hope adjusts the voltage, but im not sure.
Anyway, do you think this one is sufficient for a small test setup? just until i get a stronger one, like 10 or 12kV ?
I got the drill bits, and i drilled two 6.2 mm holes in the bottom of a large glass jar. I am thinking about doing two more for the electrodes, but
if i can come up with a better way i will skip that.
If the jar doesnt hold up to the temperature i will use terracotta flower pots.
I am going to get tungsten welding sticks.
If you dont count the bubblers, are there something more that i need to complete the setup?
[Edited on 6-11-2015 by antimon]
|
|
|
Texium
|
Thread Moved
22-11-2023 at 19:47 |
| Pages:
1
2 |