| Pages:
1
..
11
12
13
14
15
..
33 |
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
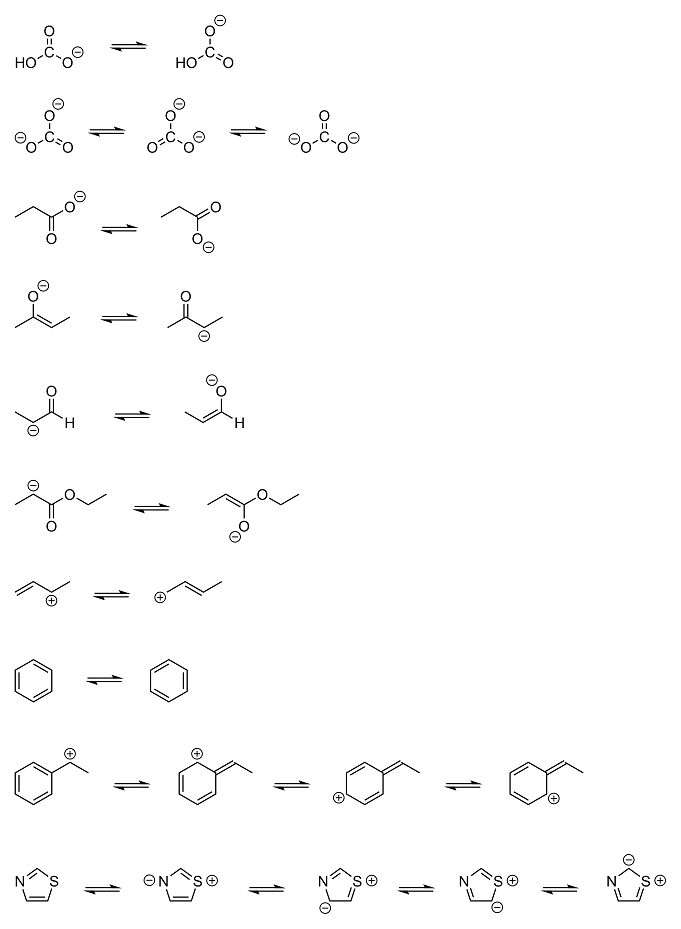
I made a few more examples of resonance structures to help further reinforce the concept. It will be important later on in your OC journey that you
understand the idea behind resonance stabilization, as it will help explain why certain structures are more stable than others; for example, why
benzyl carbocations (second to last structure) are so much more stable than most other carbocations, even when the carbon at the benzylic position is
primary. This will also help you understand why certain reactions work on one molecule and not on another, and why one pathway is sometimes preferred
over another. (there are obviously many other factors that come into play as well, for instance steric hindrance, but you have to take it one step at
a time. these concepts will all add up in the end)
Anyway, most of these should be pretty straightforward. I didn't show any resonance structures involving radicals, but they can also be
resonance-stabilized in a similar fashion. The only one that might be a little confusing is the first step of the last example (thiazole). Just keep
in mind that sulfur, like oxygen, has a third lone pair of electrons that can form bonds. So in that first step, sulfur's lone pair comes down and
bonds with carbon, breaking the pi bond between nitrogen and carbon and forming a charged sulfonium ion (the sulfur equivalent to an oxonium ion) that
gets countered by the negative charge it creates on nitrogen.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | Odd means No when it's σ, except when it's π because then it's Yes! |
OK. So Not σdd and Not eveπ. Thanks.
Resonance seems OK too.
That probably means that i'm missing something about it.
Thanks for continuing to explain all this. It is very much appreciated.
(p.s. what do you use for drawing the OC diagrams ?)
[Edited on 8-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Thanks for continuing to explain all this. It is very much appreciated.
(p.s. what do you use for drawing the OC diagrams ?)
|
Two more instalments (I might cram them into one, actually), let me know when you're ready.
Then it's time for torture (Mwhahahhahahahhhaaa!):
https://youtu.be/Tym0MObFpTI
PS: I wonder about that software too...
[Edited on 8-10-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
ChemDraw Pro. Accept no substitutes.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Cost?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Website : http://scistore.cambridgesoft.com/DesktopSoftware/ChemDrawPr...
says $190 a year
For me, that's $190 per drawing !
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
And you won't find a knock off on the 'Darknet' either! 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Unleash the Quantum Beast.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
When a Covalent Bond is not a Covalent Bond Anymore:
So far we’ve dealt only with covalent bonds, in which one or more molecular orbitals between the bonded nuclei form a stable ensemble. We’ve also
seen that covalent bonds are often permanently polarised and in this section we’ll explore bonds that are ‘extremely’ polarised.
Below is a plot of the boiling points (BP) of the chlorides CCl<sub>4</sub>, BCl<sub>3</sub>, BeCl<sub>2</sub> and
LiCl versus the difference in electronegativity of the constituent elements (e.g. Cl = 3.2, C = 2.6, difference = 1.6):
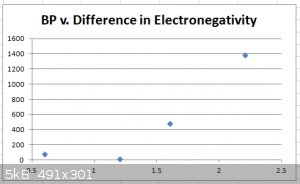
The trend is clear: higher difference in EN correlates with higher BP. This is largely (but not only) due to bond polarisation.
While CCl<sub>4</sub> and BCl<sub>3</sub> are rightly considered covalent compounds, clearly LiCl isn’t: it’s an ionic
compound. BeCl<sub>2</sub> positions itself somewhere between covalent and ionic.
Now look at the case of NaCl.
Na valence electron: 3s<sup>1</sup>
Cl valence electrons: 3s<sup>2</sup> 3p<sup>5</sup>
Bonding could seemingly occur between the Na 3s<sup>1</sup> and a Cl 3p<sub>x</sub><sup>1</sup> electron,
forming a sigma s-p bonding molecular orbital. However, the difference in electronegativity between Na and Cl is very large and as a result the
bonding MO actually resides on the Cl atom entirely, with Na having lost its 3s1 electron in permanence. Na has become Na<sup>+</sup> and
Cl has become Cl<sup>-</sup>. Electrostatic attraction between both ions is an ionic and not a covalent bond. This simple model for NaCl
(and other ionic compounds) isn’t very realistic (no covalent bonds form) and in the last chapter we’ll spend a little time on a more appropriate
model.
We can nonetheless conclude the following:
'Covalent' and 'ionic' are relative to each other, with many compounds (or bonds) somewhere between the two extremes. The extremes can be represented
by H<sub>2</sub> (no difference between the electronegativities of the bonded atoms at all) and CsF (maximum difference between the most
electronegative atom F and the least electronegative atom Cs).
In that respect, NaCl is ionic but CsF is even more ionic!
[Edited on 9-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
!
Brain Fused.
Will soak it overnight in EtOH and see if it can absorb that in the morning.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Did the soaking bring any clarity, or are clarifications required, Squire?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Maybe, let's see.
Electronegativity is a measure of how strongly an atom's nucleus attracts electrons.
(unsure whether this is independant of the atom's oxidation state/current electron count or configuration)
Bonds are formed by electrons occupying bonding orbitals between atomic nucleii.
The difference in electronegativity of the involved atoms causes the electrons to spend more time around the nucleus with the largest
electronegativity.
If that difference is very small the resulting molecule isn't polarised much.
If it's a very large difference the molecule is so highly polarised that it's called an ionic bond instead of covalent.
(does the electron truly get 'ripped off' of the Na in the case of NaCl ?)
Oh ! Covalent ! Each electronegativity is close in value. Ahhhh.
The B.P. is generally proportional to the polarisation of the molecule.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Maybe, let's see.
Electronegativity is a measure of how strongly an atom's nucleus attracts electrons.
(unsure whether this is independant of the atom's oxidation state/current electron count or configuration)
Bonds are formed by electrons occupying bonding orbitals between atomic nucleii.
The difference in electronegativity of the involved atoms causes the electrons to spend more time around the nucleus with the largest
electronegativity.
If that difference is very small the resulting molecule isn't polarised much.
If it's a very large difference the molecule is so highly polarised that it's called an ionic bond instead of covalent.
(does the electron truly get 'ripped off' of the Na in the case of NaCl ?)
Oh ! Covalent ! Each electronegativity is close in value. Ahhhh.
The B.P. is generally proportional to the polarisation of the molecule. |
Yup. You got it. 'Star student of the week badge' is in the post.
And yes, sodium loses its valence electron entirely to chlorine. Subject of next and LAST session. Glory, glory hallelujah.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Born-Haber Thermochemical cycle: Ionisation Energy, Electron Affinity and Lattice Energy:
To understand the forming of ionic bonds better, let’s take the Born-Haber thermochemical cycle for a typical binary ionic compound. It represents
the stages of the reaction:
M(s) + n/2 X<sub>2</sub>(g) === > MX<sub>n</sub>(s)
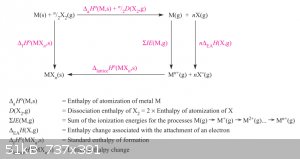
Start at the top left, from the elements.
First phase:
M(s) === > M(g): vapourisation of metal M.
n/2 X<sub>2</sub> === > n X(g): dissociation of X<sub>2</sub> molecules into X atoms.
Second phase:
M(g) === > M<sup>n+</sup>(g) + n e<sup>-</sup>: ionisation of metal gas atoms to metal gas cations.
n X(g) + n e<sup>-</sup> === > n X<sup>-</sup>(g): ionisation of X atoms to X<sup>-</sup> gas anions.
Third (and final phase): condensation of the ionic gas to the solid ionic salt.
Each step is accompanied by relevant Enthalpies, marked in red. Added together with Hess’ Law the Enthalpies of the three phases give the
Enthalpy of Formation ΔH<sub>f</sub><sup>0</sup> of MX<sub>n</sub>(s).
Let’s look specifically at the Second Phase again.
M(g) === > M<sup>n+</sup>(g) + n e<sup>-</sup> is the ionisation Enthalpy of M(g). In reality there are n ionisation
energies: 1<sub>st</sub>: M === > M<sup>+</sup> + e, 2<sub>nd</sub>: M<sup>+</sup> === >
M<sup>2+</sup> + e, etc etc. These Enthalpies become progressively larger with more electrons removed: it costs more energy to remove an
electron from a positively charged ion than from a neutral atom.
These could also be considered the oxidation energies in the gas phase but mustn’t be confused with oxidation potentials gleaned from SRP tables.
X(g) + e<sup>-</sup> === > X<sup>-</sup>(g)
The Enthalpy released when, say a gaseous halogen captures an electron is known as the electron affinity. Although related to the electronegativity
value it not identical to it. Electron affinity is a real energy but electronegativity is an empirically derived parameter but it’s not an energy.
Lattice Enthalpy:
Below are two representations (‘packed spheres’ and ‘ball and stick’) of the cubic ionic lattice of NaCl (the simplest of all ionic lattices).
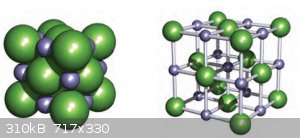
Simply put, the lattice Enthalpy of NaCl can be represented by:
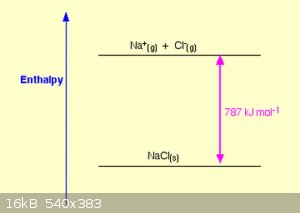
The change in Coulombic energy when the M<sup>n+</sup> ions and the X<sub>-</sub> are brought from infinity (the gas) to their
position in the lattice and is given by equation:

Which can be summarised as:

The Madelung constant is lattice dependent and tabled values can be found easily.
Understand that ionic compounds aren’t made of MX<sub>n</sub> molecules but of vast ionic lattices. There are no covalent bonds between
the constituent ions.
[Edited on 11-10-2015 by blogfast25]
[Edited on 11-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Gulp !
[Edited on 12-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Trouble in paradise?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Gimme a sleep cycle and it'll all be assimilated.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Let me know of any problems, I know it's been the end of a long, hard slog. Well done for staying the course! 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quick Navigation (Final version):
Part I – Basic Wave Mechanics
Part II - Applications of Wave Mechanics in Chemical Bond Theory
Additional Contributions
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Two fermions walk into the Pauli Bar, for few jars.
After a few too many, says the first one: 'Boy, my head is spinning!'
Asks the second one, all concerned: 'Up or down?!'
+++++++++
So, are we nearly ready for the 'Big QM/WM Quiz'?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
As ready for the Big Quiz as i'll ever be !
(so long as calculators and downright cheating are allowed)
[Edited on 18-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
As it's an 'open book' affair, I can't prevent cheating.
I have 10 questions and will post 2 per session. Participants will U2U their answers, expiry date for answers for each set of 2 is 1 week. The correct
answers will then be published.
Participant answers will be marked and a tally kept.
The first 2 questions will now be published (next post).
Good luck!
[Edited on 18-10-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Question 1: (for 10 points)
For a given particle in a one dimensional infinite potential well, the ground state energy E<sub>1</sub> is 20 eV. What is the energy used
or released to transit from the second to the third excitation?
**********************************
Question 2: (for 10 points)
The energy level of an electron in a hydrogen 2s (n = 2, l = 0) orbital is – 3.4 eV. What is the energy of that electron in a 2p (n = 2, l = 1)
orbital?
[Edited on 18-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
But ... if the answers have to be U2U'd that means no Copying either !
You're making this hard on us lying cheating weasels.
Edit:
As it's 10 points per question, can i assume that the workings out are required as well to get the full 10 points ?
[Edited on 18-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | But ... if the answers have to be U2U'd that means no Copying either !
You're making this hard on us lying cheating weasels.
Edit:
As it's 10 points per question, can i assume that the workings out are required as well to get the full 10 points ?
[Edited on 18-10-2015 by aga] |
Unless you know how to hack the U2U.
Workings out, yes, where needed. Partial answers, even wrong ones, will get credit for valiant attempts with reasoning.
1 member supplied 2 correct answers so far (20/20).
|
|
|
| Pages:
1
..
11
12
13
14
15
..
33 |