| Pages:
1
..
10
11
12
13
14
..
33 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
exam ? Exam ? EXAM ?!?!
Eeeeek !
Where to begin? The square hippopotamus is equal to the sum of the bowels of the quantum well.
Oh deary me.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | exam ? Exam ? EXAM ?!?!
Eeeeek !
Where to begin? The square hippopotamus is equal to the sum of the bowels of the quantum well.
Oh deary me. |
Of course you could waste the course money (sorry, no refunds!) and throw your life away but you don't want to do that, right?
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by aga  | | So the atoms really do have an 'effective' charge (as seen from the angle at which bonding takes place) that is not actually 1, although overall,
mathematically, taking the system as a whole, it is 1. |
As long as it helps you understand better, I suppose you could look at it that way. Just keep in mind that, yes, it is the same -1 charge
overall. The only thing changing between fluoride and iodide is the area the charge is distributed over, which does effectively make a difference.
It's kind of like taking a pencil and placing it in your palm eraser-end down and setting a heavy textbook on top of it. It doesn't hurt or break your
skin, right? Okay, now take the pencil and flip it over, putting the sharp end down into your palm. Now place the same heavy textbook on top of it.
This time it'll hurt and likely puncture the skin. In both cases the force exerted onto your palm was the same since the weight of the textbook never
changed; however, what did change was the area over which that force was distributed. A similar thing is going on with fluoride and iodide, only it's
charge and not force. The smaller the area, the stronger and more dense the charge will be at any given point within the area.
Now try to apply this to organic chemistry, for example to see why carboxylate ions are much weaker bases than alkoxide ions despite them both having
the same overall charge of negative one. I've made the following for you:
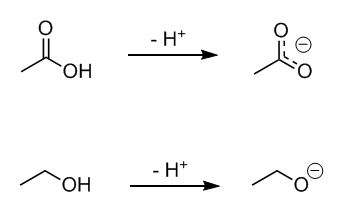
While I'm sure you're well aware, the top one is acetic acid and the bottom one is ethanol. Now let's remove a proton to give an acetate ion and an
ethoxide ion, respectively. I don't know if you've learned about resonance yet, so the dashed lines on the acetate ion may not make much sense, but
let's just say that the lines indicate that the charge is distributed evenly across the two oxygen atoms. Unlike the ethoxide ion (bottom) whose -1
charge is more or less centered completely on the one oxygen, in acetate each oxygen really only has a -1/2 charge. So while those charges may add up
to an overall charge of -1, you could say that, effectively, acetate's -1 charge is not nearly as strong as ethoxide's -1 charge, even though
overall they have the same numerical value.
Make sense?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
For aga's benefit (and because we haven't done much with resonance structures), in the top right structure of the carboxylate ion, one orbital (2
electrons) 'stretches' from one oxygen atom to the other, that 'stretched orbital' is represented by the dotted line.
This has two consequences:
1. Both C-O bonds are identical.
2. The unit charge of -1 is distributed over that O-C-O sequence, so that each O atom has a charge that is really only about -1/3. That makes them
less attractive to electrophiles like the proton H<sup>+</sup> and thus weaker bases.
A corollary is that simple alcohols are extremely weak acids, while carboxylic acids are only weak acids, much stronger than alcohols.
[Edited on 5-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So ..... would it be right to imagine that the electron on the O-C-O structure is whizzing about in a New Orbital, common to all three atoms, or is
leaping from one atom's discrete orbital to another ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | | So ..... would it be right to imagine that the electron on the O-C-O structure is whizzing about in a New Orbital, common to all three atoms, or is
leaping from one atom's discrete orbital to another ? |
Personally I prefer to think of the dotted line as a molecular orbital (TWO electrons) stretching from one O to the other O.
[Edited on 5-10-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by aga  | | So ..... would it be right to imagine that the electron on the O-C-O structure is whizzing about in a New Orbital, common to all three atoms, or is
leaping from one atom's discrete orbital to another ? |
I look at it as the electrons in the pi bond becoming delocalized. In other words, the double bond that used to be localized in acetic acid between
carbon and oxygen has now become delocalized and stretched across all three atoms. You'll learn later that this is a result of resonance
stabilization, as you could represent the acetate ion with a negative charge on either oxygen simply by alternating which oxygen has the double bond
between it and carbon. In reality, however, the bond isn't actually alternating or "resonating," which is why the more correct way to draw an acetate
ion is as a hybrid of the two structures.
[Edited on 10-6-2015 by Darkstar]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | You'll learn later that this is a result of resonance stabilization, as you could represent the acetate ion with a negative charge on either oxygen
simply by alternating which oxygen has the double bond between it and carbon. In reality, however, the bond isn't actually alternating or
"resonating," which is why the more correct way to draw an acetate ion is as a hybrid of the two structures.
|
For the reason you outlined in your last paragraph, I wasn't planning on writing on "resonance" (I didn't in the segment on conjugated bonds and
benzene either), so in that sense there will be no 'later'. If you wish to spend a few words on it then feel free (of course) and I'll include it in
the final table of contents (as well as any other additional material).
I see Feynman still refers to resonance on benzene.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I didn't necessarily mean later in this course, just later in his journey of learning organic chemistry. A brief segment on resonance does sound like
a good idea, though. I may add something about it soon.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks for the clarifications.
The notion of delocalisation seems straightforward - the electron(s) are not centred around 1 nucleus, they're centred around 2 or more.
Imagining it in simple terms of the orbital volume, it seems logical that those delocalisd electrons are less likely to become involved with an atom
external to that system, as they are not as concentrated in the 'bonding area' as they would be if they were concentrated into a smaller orbital.
Would 'resonance' be the notion of how the delocalised orbital Looks, and which atoms are involved ?
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by aga  | | Would 'resonance' be the notion of how the delocalised orbital Looks, and which atoms are involved ? |
I made this to show you what's going on when acetic acid is deprotonated. The red arrows represent the flow of electrons:
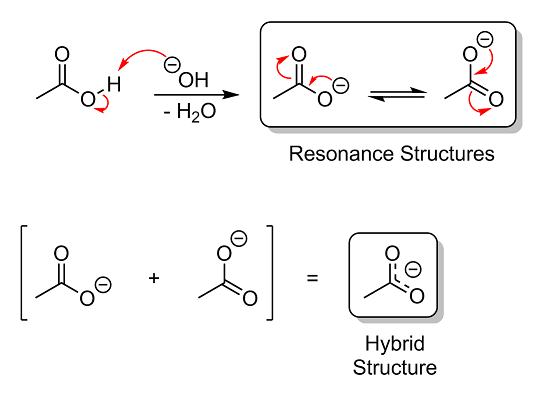
As you can see, you could draw the acetate ion with a negative charge on either oxygen simply by moving the double bond. These two structures are
called resonance structures, as they give the appearance that the molecule is "resonating" back and forth between the two. In reality, the molecule
isn't actually going back and forth, and at no time is the double bond ever truly localized between any two atoms. Instead, the molecule always exists
as a single structure, basically a combination of all the possible resonance structures. This combination is called a hybrid structure, and is the
more correct way to depict the molecule.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Again for the benefit of aga, I've 'zoomed in' on the left hand top part of the diagram, showing also the three lone pair (non-bonding) electron pairs
(orbitals) on the O-nucleus of the OH<sup>-</sup> anion:
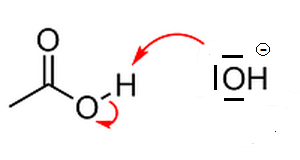
One of these lone electron pairs latches on to the H atom of the acid and that H atom then let's go of the MO that bonded it to the acid. The result
is a water molecule and a carboxylate ion.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So 'resonance' is basically seeing that the resulting structure can flip between two or more states, kind of 'oscillating' back and forth (although it
isn't).
That's kind of what i meant about the bonding orbital shape, although i didn't explain what i meant very well.
I suppose the $1m question is why does the H prefer to be with the OH- so much that it releases it's bonding with the C-O in order to do so.
Are the orbitals involved in the OH different to those in the C-O-H (it's an O to H bond after all in both cases).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
I suppose the $1m question is why does the H prefer to be with the OH- so much that it releases it's bonding with the C-O in order to do so.
|
That's really a matter of molecular orbital energy levels, kind of the subject of the next instalment, coming up in about 15'.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Bond Enthalpies:
So far I’ve avoided using molecular orbital diagrams, writing mostly from VSEPR point of view. But let’s go back to the beginning of Part II, in
particular the interaction between two ground state hydrogen 1s<sup>1</sup> orbitals to form a bonding σ MO. In Molecular Orbital Theory
this is often schematised as follows in an MO diagram:
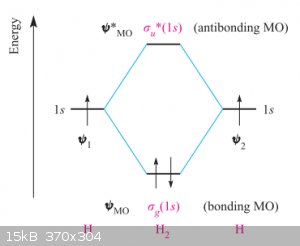
(if you’re wondering what the u and g symbols stand for, u stands for ‘ungerate’ which is German for ‘odd’
and in the parlance of this course means ‘out of phase’. g stands for ‘gerate’, which is German for ‘even’ and in the parlance of this
course means ‘in phase’.)
One of the beauties of the MO diagram is that it shows the relative energy levels of the orbitals involved. We can see that energy of the filled
bonding MO, noted as σ<sub>g</sub>(1s), is lower than the energies of the atomic orbitals ψ<sub>1</sub> and
ψ<sub>2</sub> of the individual H atoms. The anti-bonding MO σ<sup>*</sup><sub>u</sub>(1s) remains of course
empty.
In practice this means that the reaction:
H. + .H ===> H - H (or 2 H === >H<sub>2</sub>
... is exothermic, i.e. ΔE < 0. ΔE is the bond enthalpy of the formation of the σ<sub>g</sub>(1s) MO bonding orbital.
Bond enthalpies for the main types of covalent bonds between various atoms have been experimentally determined and a sample of values is shown below:
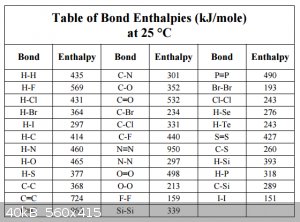
Note that in the case of formation of bonds, we have to assign a negative sign to these values, in the case breaking bonds a positive sign has to be
assigned.
Furthermore, while the bond enthalpies between monovalent atoms are absolutes, for bonds between multivalent atoms the real bond enthalpy may vary a
bit from context to context: a C-C bond in ethane is not exactly the same as in butane (e.g.). In that case the bond enthalpies have to be considered
approximate, average values.
Estimating reaction Enthalpies from bond Enthalpies:
These values can be used to estimate the reaction enthalpies of simple reactions. Here, mainly for illustrative purposes, the estimated Enthalpy of
formation of ethane:
2 C + 3 H<sub>2</sub> === > C<sub>2</sub>H<sub>6</sub>, ΔH<sub>f</sub><sup>298K</sup>
To find this value write this equation as a sum of ‘sub reactions’ using Hess’ Law:
1) 2 C === > C – C, yields - 368 kJ
2) 3 X [H<sub>2</sub> === > 6 H.], costs 3 X + 435 = + 1305 kJ
3) C – C + 6 H. === > H<sub>3</sub>C – CH<sub>3</sub>, yields 6 X – 414 = - 2484 kJ
Adding up: ΔH<sub>f</sub><sup>298K</sup> = - 368 + 1305 – 2484 = -1547 kJ per mol
C<sub>2</sub>H<sub>6</sub>. NIST webbook lists the value of ΔH<sub>f</sub><sup>298K</sup> as – 1560
kJ/mol, about 1 % difference.
Note that where phase changes happen, e.g. had ethane been a liquid at 298 K, a correction would have to be applied for the phase change enthalpy but
that was not the case here.
Further simplified this method can also be used for the estimation of reaction Enthalpies of simple substitutions. Take the substitution of a single
hydrogen atom in an alkane molecule by a chlorine atom:
C<sub>n</sub>H<sub>2n+2</sub>(g) + Cl<sub>2</sub>(g) === >
C<sub>n</sub>H<sub>2n+1</sub>Cl(g) + HCl(g)
To effectuate this we need to:
1) break 1 mol of C-H bonds, which costs + 414 kJ
2) break 1 mol of Cl<sub>2</sub> bonds, which costs + 243 kJ
3) form 1 mol of C-Cl bonds, which yields – 331 kJ
4) form 1 mol of H-Cl bonds, which yields – 431 kJ
Which gives a total of -105 kJ/mol of estimated reaction enthalpy at 298 K. In reality that value will depend a bit on the alkane, the substitution
position and possible phase changes.
And with this we’ve arrived at something really quite fundamentally important: chemical reaction Enthalpies are the consequence of the
breaking and forming of molecular bonds, when molecular orbitals rearrange into energetically lower (more favourable) configurations.
[Edited on 7-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Jees bloggers ! That's a lot of bits all at once !
Firstly the odd/even diagram thing : Bonding / Anti-bonding is unclear to me, unless it just means that a Bond is formed when the resulting system is
at a Lower energy state than it would be otherwise (edit: for a given Energy level).
Is this the Entropy thing where stuff always goes from a High to Low energy state if it can ?
[Edited on 6-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Jees bloggers ! That's a lot of bits all at once !
Firstly the odd/even diagram thing : Bonding / Anti-bonding is unclear to me, unless it just means that a Bond is formed when the resulting system is
at a Lower energy state than it would be otherwise (edit: for a given Energy level).
Is this the Entropy thing where stuff always goes from a High to Low energy state if it can ?
|
Bonding / anti-bonding reminder:
http://www.sciencemadness.org/talk/viewthread.php?tid=62973&...
Yes, the system H<sub>2</sub> is of lower energy than the system of H. + .H (H. is an unbonded hydrogen atom), that's what the diagram
shows. The difference ΔE is the bond Enthalpy, literally the energy released when the bond forms. We can even write ΔE more
explicitly as:
ΔE = E<sub>σ</sub> - 2 E<sub>ψ</sub>
Entropy? Huh? Who mentioned that? 
[Edited on 7-10-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Another simple example demonstrating the use of bond enthalpies.
Estimate the Enthalpy of formation of water, ΔH<sub>f</sub><sup>298K</sup>.
H<sub>2</sub>(g) + 1/2 O<sub>2</sub>(g) === > H<sub>2</sub>O(l)
We're only interested in Enthalpy, so thermodynamics, and not in kinetics or how precisely we carry out this reaction. But we know for certain that it
involves:
1) Breaking 1 mol of H<sub>2</sub> bonds: H<sub>2</sub>(g) === > 2 H(g). This costs + 435 kJ.
2) Breaking 1/2 mol of O<sub>2</sub> bonds: O<sub>2</sub>(g) === > 2 O(g). This costs 1/2 X + 498 = + 249 kJ.
3) Forming bonds 2 mol of HO: 2 H(g) + O(g) === > H<sub>2</sub>O(g), This yields 2 X - 465 = - 930 kJ.
- 930 + 249 + 435 = - 246 kJ/mol. The NIST webbook is - 286 kJ/mol but that is for liquid water. So to our value we have to add the Latent Heat of
Condensation of -41 kJ/mol, so we get - 287 kJ/mol. Not too shabby!
[Edited on 7-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Wow !
So the data works out almost perfectly.
Would this be a bad time to ask what Energy actually is ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
So the data works out almost perfectly.
Would this be a bad time to ask what Energy actually is ? |
It's not hard to find some examples where it doesn't work so sterlingly. But for reasonable estimates 'playing Lego' with bond Enthalpies works well.
Energy: for all intents and purposes practical:
Something has energy if it has the capacity to do useful work.
Useful work in the physics sense of the word:
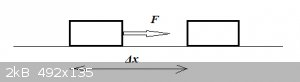
The force F displaces a object by a distance Δx in the same direction as F. The useful work done on the object is:
W = FΔx, assuming F was constant over the interval Δx.
If F is not constant then:
W = ʃF(x)dx, integrated from x = 0 to x = Δx.
Example: in internal combustion engines chemical energy of the fuel and air is converted to combustion Enthalpy, which is in turn used to drive the
piston upward and do useful work on it.
We now know that 'Molecular Orbital Energy' would be a better term for chemical energy!
[Edited on 7-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Valliant effort, and gratitudes for it.
The actuality of Energy Itself might lay in a distant corner of unexplored agaspace.
Who knows ? Back to mainstream and safer ground ...
Is the out-of-phase antibonding orbital result of the wavefunction predicting that a bond will NOT form, or is it called 'antibonding' to denote that
a bond Might form, wheras the bonding orbital (in-phase) denotes that a bond Will form ?
Just that the word 'antibonding' suggests that a bond will not form.
[Edited on 7-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It means that no bond is formed. In an anti-bonding molecular orbital the bulk of the electron density doesn't lie between the nuclei and provides no
shielding against the electrostatic repulsive forces between the nuclei: that's a non-bonding arrangement.
Example: σ<sup>*</sup><sub>u</sub>(1s) representation, scroll down and see how one orbital is red (+) and the other blue (-),
they are ungerate:
http://winter.group.shef.ac.uk/orbitron/MOs/H2/1s1s-sigma-st...
[Edited on 7-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So odd means No.
Antibond means No Bond Forms.
Out-of-phase means No bond.
OK. Got it.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, correct.
Ahem. Cough. There's a small complication. For π bonds it's the opposite: ungerate orbitals form bonding MOs,
gerate orbitals form anti-bonding orbitals.
I'll now dedicate a mini-excursion on that gerate/ungerate business. Need to make a diagram. Bear with me.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Gerate and ungerate orbitals:
Gerate/ungerate relates to the concept of wave function parity we discussed in Part I.
Look at the diagram below. In it orbitals or lobes of orbitals are coloured red or blue, corresponding to the sign of the wave function, + or - (or
the other way around).
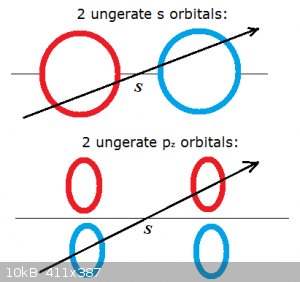
Whether the orbitals are gerate or ungerate is determined as follows.
Draw an arrow through any point of symmetry:
If the colour (sign) doesn't change when crossing the symmetry point (S) the lobes are gerate.
If the colour (sign) does change when crossing the symmetry point (S) the lobes are ungerate.
In the diagram the top s orbitals are ungerate and form anti-bonding σ molecular orbitals.
The bottom p<sub>z</sub> are also ungerate but form bonding π molecular orbitals.
What you really need to take home from this:
1) Bonding σ molecular orbitals are formed from the interaction of gerate (g) s or p<sub>x,y</sub>
orbitals.
2) Bonding π molecular orbitals are formed from the interaction of ungerate (u) p<sub>z</sub> orbitals.
To paraphrase:
Odd means No when it's σ, except when it's π because then it's Yes!  
Und das ist ze geschichte der gerate und ungerate, Herr Aga! Ein bier, bitte... 
[Edited on 8-10-2015 by blogfast25]
|
|
|
| Pages:
1
..
10
11
12
13
14
..
33 |