| Pages:
1
..
23
24
25
26
27
..
81 |
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Agreed there. The precursors are easy to get and very little skill or knowledge is needed to make acetone peroxide the synthesis is widely published
and common on many how to sites and the yields are good so why not? Also the younger more inexperienced really have no idea on just how bad an
accident with this stuff could be. younger people do take more risks. See that video of the Indian kid pressing 1g of peroxide in foil with his bare
hands? If he really knew what he was doing he would not be doing that.
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
I accidentally posted twice the exact same post by resendjng the same info but I can't seem to delete it.
so can a mod pls remove it?
|
|
|
Goshunkar
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Hello,
I would like to test this receipt :
Do you think it would explode (with a small booster and confinement from a steel pipe) ?
AN-Gel :
Ammonium Nitrate 250g 55%
Potassium Nitrate 45g 10%
Aluminum powder 68g 15%
Sugar 23g 5%
Guar Gum 11g 2.5%
Borax 4.5g 1%
Water 68g 15%
Can water be substituted with H2O2 (50% solution)? If so, is the resulting explosion more powerful ? And can the gel be stored for a long time (with
H2O2) ? What is the function of the Borax powder and KNO3 ?
I would also like to test a mixture I read about here :
http://www.sciencemadness.org/talk/viewthread.php?tid=3214&a...
H2O2 - glycerine
Since I can buy H202 (50% solution) cheaply , this is interesting. In the attachment of user jpsmith123 : http://www.sciencemadness.org/talk/files.php?pid=59003&a... ; I read this line in the table : H202 : 34% ; glycerine : 15% ; water 51 %.
But does 'do' mean that it actually exploded ?
If so : would 85 % of H2O2 (50% solution) and 15% glycerine be a good cap sensitive explosive ?
[Edited on 23-9-2015 by Goshunkar]
|
|
|
bb911gt4
Harmless

Posts: 17
Registered: 25-12-2013
Member Is Offline
Mood: No Mood
|
|
Where can I get this igniter (or make it)
Hey guys,
Have you ever seen these igniters?
https://www.youtube.com/watch?v=VWcAjHuS1L4
Any idea where I can get them or how they're made? It looks like a coil of wire that is pulled through another piece of metal. What chemicals are
used?
I would prefer to buy them pre-made but I would could make them as well.
Thanks!
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
A friction sensitive pyrogen mixed with adhesive and applied to a potion of the string. This segment is pulled through a tight, abrasive opening,
which ignights it, and pulled it into a small charge of a hot burning or spark throwing pyro mix. The flames amd sparks are directed away from the
string puller. If I were to guess.
Similar, in a way, to those novelty poppers that have a pull strings on either side.
[Edited on 24-9-2015 by Bot0nist]
You could probably rig it with match heads, and the striker portions of the box. A slow burning black powder or rcandy could work as the fire starter
mix.
[Edited on 24-9-2015 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Bert
|
Threads Merged
24-9-2015 at 07:30 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Firefox sells kits for making this type of igniter. Paint ball players use such to ignite smoke devices, among other things.
http://www.firefox-fx.com/kits.htm
This general type of pull wire/pull string igniter has been in common use since Potassium chlorate became available to pyrotechnists- Used to ignite
everything from USA civil war era muzzle loading cannons to Vietcong hand grenades. A look at the Tenney Davis COPAE book in the forum library may
disclose mixtures.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Bert  | The origin of this question is some comments in Naoum on use of high density oxidizers for dopes in low NG wartime mining explosives (dynamite dope
formulations): For example, the higher VOD resulting from density change when substituting KClO4 for NH3NO3 offsetting the lowered VOD otherwise
caused by a very low NG content.
Off the top of your head-
What are the highest density choices available among inorganic perchlorates, nitrates or chlorates, qualifying as being of a reasonably non
hygroscopic nature... That is, compounds that one could leave a sample of sitting exposed to air for several days at STP and around 90% relative
humidity, and not have it convert itself into a puddle!
Extra points for compounds that are reasonably stable in storage from -40 to at least 80C., and don't present a horrible handling problem due to
toxicity, corrosive/reactive towards common engineering materials, etc.
Industrial choices of oxidizers in military and civil pyrotechnics typically being driven by OTHER considerations than density (such as economics),
what if we think outside the box...
Google has not been my friend (so far) on this question.
(Edit)
My first thought was Barium nitrate and perchlorate at densities around 3.29 & 3.2, respectively. But a bit toxic-
[Edited on 19-9-2015 by Bert] |
This question is a hard one!
NG is already over-oxygenated so adding oxydiser will make little use if the rest of the "dopes" is not a fuel.
Assuming it is a fuel, ideally it should be explosive, high density and with a negative OB...then adding an oxydiser will maybe enhance the explosive
power or at least allow it to remain stable despite being lower at NG and explosive fuel.
This effect is seen with Ammatols (mixes of NH4NO3 and TNT), Baratols (mixes of Ba(NO3)2 and TNT), Sodatols (mixes of NaNO3 and TNT). The
"inexpensive"/"cheap" oxydiser allows to get nearly the same explosive characteristics as plain TNT...the oxydisers increases the heat output by
burning the fuel available of the negative OB part of the TNT.
The question is not easy because high density negative OB explosives like TATNB mixed with NH4ClO4 yield a mix that is beneficial to each other
(synergic) and result in higher detonic performances than each explosive material appart/alone (near the 9500 m/s VOD for the mix).
But in the case of oxydisers with a metal (heavy), not only the ratio of the amount of oxygen weight vs the metal dead weight is important... for
example Al(ClO4)3 would be better than Mg(ClO4)2 itself better than NaClO4; but it is not clear if the mass of the metal dead weight may have a
detonic impact on VOD and brisance or if the benefit only comes from the added burnt fuel and overal energy output...normaly it should be detrimental
to the exhaust gas speed (what is enhanced by light gases).
A simple comparison between several stoechiometric mixes may give a precise answer to this... vs TATNB or TNT and vs each other...
TATNB or TNT / LiClO4
TATNB or TNT / NaClO4
TATNB or TNT / KClO4
TATNB or TNT / NH4ClO4
TATNB or TNT / Mg(ClO4)2
TATNB or TNT / Ca(ClO4)2
TATNB or TNT / Sr(ClO4)2
TATNB or TNT / Ba(ClO4)2
TATNB or TNT / Al(ClO4)3
TATNB or TNT / Ni(ClO4)2
TATNB or TNT / Ni(ClO4)3
TATNB or TNT / LiIO4
TATNB or TNT / NaIO4
TATNB or TNT / KIO4
TATNB or TNT / NH4IO4
TATNB or TNT / Mg(IO4)2
TATNB or TNT / Ca(IO4)2
TATNB or TNT / Sr(IO4)2
TATNB or TNT / Ba(IO4)2
TATNB or TNT / Al(IO4)3
TATNB or TNT / Ni(IO4)2
TATNB or TNT / Ni(IO4)3
This should prove the effect or not:
-of heavier metal (Li vs Na vs K ; Mg vs Ca vs Sr vs Ba)
-of heavier non-metal (Cl vs I)
-of higher valence metals, thus increasing OB of the oxydiser
(Na vs Mg vs Al; Al vs Ni(III); Ni(II) vs Ni(III))
(stoechiometry would thus need proportionally less oxydiser (by weight) vs explosive fuel)
Quote: Originally posted by Bert  | Looking at the Wikipedia article (lazy!), I see a density of 3.327 g/cc for Cesium perchlorate vs. 2.52 for KClO4. Also an even lower water
solubility than KClO4 AND decomposition temperature of 250 C, compared to the 400 C and up for KClO4...
Have not yet looked at the reactivity and prices and so shattered my illusions.
A recently linked article in Axt's OTC pentryl article mentions the use of a percentage of KClO4 added to boosters made with pentryl as further
enhancing pentryl's (already rather good) power, but with no specific mention of BRISSANCE. Let's daydream a bit further, perhaps there's an
application for such a mix in base charges and small boosters?
(Edit)
Pentryl has a listed density of 1.68g/cc, and an Oxygen balance of -46%. An admixture of Barium or Cesium perchlorate at over 3G/cc density should
give a decent increase in density, possibly boosting VOD/brissance and help oxidize some of that leftover Carbon for more heat output ... Where will
the "sweet spot" in performance as a booster fall in any trade off between total power output and potentially increased VOD/brissance? Enquiring
minds want to know!
[Edited on 21-9-2015 by Bert]
[Edited on 23-9-2015 by Bert] |
It is evident that if the oxydiser is already a high detonating explosive, this will kick even more the VOD and brisance.
The following are interesting cases of oxydisers because the VOD and density are high but some of them display slighly reduced performances when used
alone because strongly overoxygenated...
So probably worth a try:
TATNB or TNT / N2H5NO3 (already VOD 8900 m/s alone)
TATNB or TNT / N2H5ClO4 (already VOD 7500 m/s alone)
TATNB or TNT / N2H5IO4 (unstable?)
TATNB or TNT / N2H5C(NO2)3
TATNB or TNT / HONH2.HNO3 (already VOD 8000 m/s alone)
TATNB or TNT / HONH2.HClO4
TATNB or TNT / HONH2.HIO4 (if stable)
TATNB or TNT / HONH2.HC(NO2)3
Of course any dense nitroaromatic HE would do the job if displaying a negative OB...the denser the best...
TNB, TeNN, Tetryl, Pentryl, poly-trinitrophenylene, tri-methylnitramino-trinitrobenzene (hextryl), trimethylol-trinitrobenzene trinitrate, ...
...
[Edited on 25-9-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Goshunkar
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
No answers for me ? 
|
|
|
specialactivitieSK
Hazard to Self
 
Posts: 94
Registered: 21-10-2014
Member Is Offline
Mood: No Mood
|
|
Tested someone ammonium trichromate (NH4) 2Cr3O10. Which is formed by crystallization of ammonium dichromate nitric
acid spec. weight 1.39 g / cm3 (Siewert, 1862). This compound explodes at 190 ° C, giving rise to NO and NO2.
[Edited on 25-9-2015 by specialactivitieSK]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Goshunkar  | Hello,
I would like to test this receipt :
Do you think it would explode (with a small booster and confinement from a steel pipe) ?
AN-Gel :
Ammonium Nitrate 250g 55%
Potassium Nitrate 45g 10%
Aluminum powder 68g 15%
Sugar 23g 5%
Guar Gum 11g 2.5%
Borax 4.5g 1%
Water 68g 15%
Can water be substituted with H2O2 (50% solution)? If so, is the resulting explosion more powerful ? And can the gel be stored for a long time (with
H2O2) ? What is the function of the Borax powder and KNO3 ?
I would also like to test a mixture I read about here :
http://www.sciencemadness.org/talk/viewthread.php?tid=3214&a...
H2O2 - glycerine
Since I can buy H202 (50% solution) cheaply , this is interesting. In the attachment of user jpsmith123 : http://www.sciencemadness.org/talk/files.php?pid=59003&a... ; I read this line in the table : H202 : 34% ; glycerine : 15% ; water 51 %.
But does 'do' mean that it actually exploded ?
If so : would 85 % of H2O2 (50% solution) and 15% glycerine be a good cap sensitive explosive ?
|
1°) Glad you want to test... don't forget to report succes
And maybe tell us where you did find the receipe...
2°) NH4NO3 water gels need good detonator, confinement and also very critical...the pipe diameter...too small will lead to no detonation -->
critical diameter of detonation.
3°) H2O2 will work, but formula must certainly be adapted because of OB...more oxydiser --> more fuel needed.
H2O2 will help getting micro-bubbles what sensitize the NH4NO3 explosive gels...but it will also decrease storage stability...especially in steel pipe
(iron --> rust --> not compatible with H2O2).
4°) Borax and KNO3 are probably present for OB and for viscosity of the water gel.
5°) If the patent says so, then you must be in the sensitive triangle area (H2O2/H2O/Glycerol) (the stoechimetric line is probably the more sensitive
one)...The "do" in table 1 does indeed mean detonation/explosion.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Goshunkar
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Quote: Originally posted by PHILOU Zrealone  | 1°) Glad you want to test... don't forget to report succes
And maybe tell us where you did find the receipe...
2°) NH4NO3 water gels need good detonator, confinement and also very critical...the pipe diameter...too small will lead to no detonation -->
critical diameter of detonation.
3°) H2O2 will work, but formula must certainly be adapted because of OB...more oxydiser --> more fuel needed.
H2O2 will help getting micro-bubbles what sensitize the NH4NO3 explosive gels...but it will also decrease storage stability...especially in steel pipe
(iron --> rust --> not compatible with H2O2).
4°) Borax and KNO3 are probably present for OB and for viscosity of the water gel.
5°) If the patent says so, then you must be in the sensitive triangle area (H2O2/H2O/Glycerol) (the stoechimetric line is probably the more sensitive
one)...The "do" in table 1 does indeed mean detonation/explosion. |
Thanks for your answer.
I got the first receipt from a dubious website : http://cdn.preterhuman.net/texts/wars_and_weapons/other/Impr...
The pipe has a diameter of 5.1 cm. Wide enough ?
I will report the result.
I guess the H202-glycerine explosive has to be confined too ?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Goshunkar,
You thus forgot the Aluminium powder inside your mix...
In your link: Aluminum powder 68g 15%
Diameter 5.1cm should be enough...especially with H2O2...H2O2/Al/gel was tested by Axt in plastic bag.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Goshunkar
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Nope, I didn't forget the Al powder ...
Are you referring to http://www.sciencemadness.org/talk/viewthread.php?tid=3214 ?
He used another composition
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yes...as written earlier H2O2/Al/gel ... thus looked pretty close to what you intend to do with H2O2 since Al was listed in the reference source
receipt you provided...but not in the receipt you wrote 
BTW: Al would boost things up and sensitize the mix even more...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
So, I have what might be one of the dumbest questions I've asked in a while...... Does anyone know of any synthesis or inquiry related to the nitrites
(not nitro, NO groups) of toluene or other similar compounds (xylene, benzene) ? The closest I could find was a paper written in the late sixties in
london that was using it to figure out the stereospecificity of xylene.
As an aside, I was wondering if anyone had any solid information on the properties of nitroxylene (20ml of Xylene). I recently made some using the
commercial grade stuff (at least, I think) through solution of sulfuric acid(50ml)/KNO3(15g, way way too much). After I added the xylene it stratified
rather quickly, even when stirred. I then poured it into a separatory funnel. It stayed separated into layers and I drained off the acid into some
water very slowly (what I think is nitroxylene stuck towards the top), and was left with a milky white fluid which I poured off into an amber sample
jar and refrigerated.
I am very interested in seeing if I can find anyone who has sample to compare to or some other information on the topic. Even if it's a very wimpy
explosive I'm still somewhat interested just because of how incredibly easy it was to make this small-ish sample. 
I've exhausted my ability to find information on either at this point, I think.
|
|
|
Bert
|
Threads Merged
29-9-2015 at 17:08 |
Bert
|
Thread Split
29-9-2015 at 19:25 |
kecskesajt
Hazard to Others
  
Posts: 299
Registered: 7-12-2014
Location: Hungary
Member Is Offline
Mood: No Mood
|
|
There is a compuond called nitrosobenzene, C6H5NO.
Also, trinitroxylene is a waxy, solid material.What you made might be a mix of mononitroxylene and xylene.For the nitration of xylene you need fuming
HNO3.
|
|
|
Thanatops1s
Hazard to Self
 
Posts: 54
Registered: 24-6-2013
Member Is Offline
Mood: No Mood
|
|
So after my second attempt I had a successful DDNP synthesis. I did a quick seach but wanted a more definite answer as to what is the best method of
storage. I don't plan on storing it for an extremely long time and no more than a few grams. Just wanted to be as safe as possible. Thanks.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
I figured here was the best place for my question, so here goes.
I have almost zero interest in EM and certainly no experience. However, if aga can do organic, then I can do something combustable.
My four year old son has developed an interest in "rocket ships". I figured a quick and simple rocket in the back yard might be just the thing.
Preferably something that "squirts fire".
What I would like is a relatively safe and straightforward design and a suitable propellant mix. I have plenty of potassium nitrate that I can use.
Suggestions?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
And another pyro is born...
http://www.wichitabuggywhip.com/fireworks/
Do you want to fly, (rocket engine) or just have the device emit a jet of sparks (gerb).
Either way, a black powder type mix would be a suitable fuel. Perhaps with an additive for spark production, such as 80 mesh charcoal, steel powder or
fine Titanium sponge.
Basic device construction is the same, a tube filled with fuel. The rocket usually has a central hollow core to give a larger burning surface, the
gerb usually just burns from the end and so has a longer burn time.
http://www.wichitabuggywhip.com/fireworks/rockets/rockets.ht...
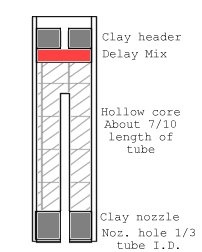
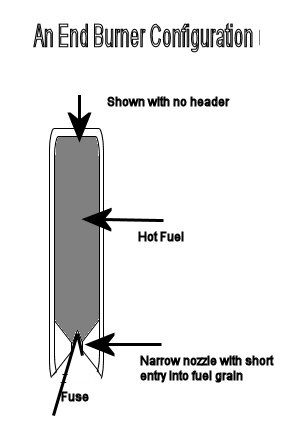
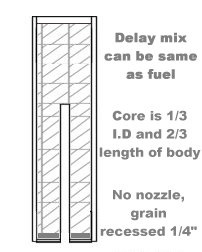
[Edited on 3-10-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by j_sum1  | I figured here was the best place for my question, so here goes.
I have almost zero interest in EM and certainly no experience. However, if aga can do organic, then I can do something combustable.
My four year old son has developed an interest in "rocket ships". I figured a quick and simple rocket in the back yard might be just the thing.
Preferably something that "squirts fire".
What I would like is a relatively safe and straightforward design and a suitable propellant mix. I have plenty of potassium nitrate that I can use.
Suggestions? |
Try this but be careful : https://www.youtube.com/watch?v=12fR9neVnS8
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
j_sum1, do you plan on actually launching a rocket or just doing a static test in the dirt for fun? I whipped up something one afternoon about a year
ago using a kitchen wrap tube capped at one end with duct tape, potassium nitrate, sucrose, a little glucose and a pinch of iron oxide. https://www.youtube.com/watch?v=x5b_uScBVJk
The footage isn’t great and I picked an overcast, rainy day to do it (was worried about fires) but you get the idea.
It was heaps of fun and I'll do it again sometime.
if you want I’ll dig up the mix ratios I used.
edit: fixed link, also, the chuffing was from the electric igniter not being pushed far enough into the core

[Edited on 3-10-2015 by NedsHead]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Thanks for the answers Bert and Ned. Since posting, I have taken a look at a couple of YT vids. This one impressed: https://www.youtube.com/watch?v=12fR9neVnS8
I was thinking more along the lines of sugar rather than black powder since I do not currently have any charcoal available. Something added to give a
bit more of a visible flame would be good. I do have Ti powder and Al powder if that helps. I have copper nitrate too which I assume would give a
green colour.
I really don't want anything too powerful. I will probably scale down pretty small. I live in a suburban area and only need a lift of 15-20 metres
maximum.
And to answer your question, NedsHead, a rocket ship has to fly. But according to the boss, "It's not supposed to go to space because that's too
far."
[edit]
missed your post, ecos. You linked the very same one vid that I found.
[Edited on 3-10-2015 by j_sum1]
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
sounds like the boss has things under control j_sum1, adding Al powder will give the rocket ship a nice glittering tail. I wouldn’t recommend using
PVC pipe as a motor casing because if the core over pressurizes and you have a CATO you will have shards of PVC flying everywhere. melt casting might
be easier than all that tamping
melt casting
https://www.youtube.com/watch?v=hyPysthIRss
[Edited on 3-10-2015 by NedsHead]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by j_sum1  | Thanks for the answers Bert and Ned. Since posting, I have taken a look at a couple of YT vids. This one impressed: https://www.youtube.com/watch?v=12fR9neVnS8
I was thinking more along the lines of sugar rather than black powder since I do not currently have any charcoal available. Something added to give a
bit more of a visible flame would be good. I do have Ti powder and Al powder if that helps. I have copper nitrate too which I assume would give a
green colour.
I really don't want anything too powerful. I will probably scale down pretty small. I live in a suburban area and only need a lift of 15-20 metres
maximum.
And to answer your question, NedsHead, a rocket ship has to fly. But according to the boss, "It's not supposed to go to space because that's too
far."
[edit]
missed your post, ecos. You linked the very same one vid that Ik found.
[Edited on 3-10-2015 by j_sum1] |
Black powder charcoal is fairly easy to achieve- My first junior high school chemistry lab was "destructive distillation of wood". And it's even
easier if you're not condensing the vapors for later analysis... A clean metal paint can or old cookie tin with a small hole punched in the bottom,
set on the BBQ grill until the smoke comin out the bottom hole stops burning. Set on dirt or sand and allow to cool completely.
Sugar rockets have approximate 1.5 times the specific impulse of old fashioned black powder, FYI! So if you WANT low performance, something to
consider there- Copper nitrate (cuprous? cupric???) may not color a Carbon rich flame too well, and AFAIK both are hygroscopic, which is a bad thing.
Copper carbonate or black Copper oxide are my usual choices in a blue flame colorant.
Try dissolving the Copper nitrate in methanol and making a "ghost mine" with it instead...
Fine Aluminum powder will probably burn inside the combustion chamber, rather than providing a silver spark "tail", Ti works better. Charcoal works
for gold spark tail, and grain size is easier to alter than Ti.
[Edited on 3-10-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Reporting back.
I mixed up a 62/38 blend of KNO3 and powdered sugar. Actually, a lot more than I intended since I inadvertently made a 38/62 blend and had to add
some more nitrate.
A test burned nice and feisty. My rocket engine design needs a bit more work. Attempting to keep things small scale, I packed the material inside
short sections of drinking straw. But, I gave little thought to the nozzle, had no decent fuse and with the narrow diameter, I did not drill a core
hole. Lots of smoke and fizzing but little in the way of flight. Turns out that Mr 4 wasn't that interested this time around anyway. Miss 7 thought
it was great. We had a good time adding various agents to the fuel mix to achieve different kinds of sparkles and colour. Ti powder was definitely
the most impressive although Zn powder was pretty cool too. (I think however the Zn was too fine.) Strontium carbonate failed to produce the red I
was hoping for and CuO didn't produce a particularly vibrant colour either.
I have quite a bit left and will attempt a casting some time -- probably with some titanium added.
|
|
|
| Pages:
1
..
23
24
25
26
27
..
81 |