| Pages:
1
..
36
37
38
39
40
..
77 |
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Here's samarium and holmium sulfamate in solution. As far as I know holmium sulfamate hasn't been characterized - Google results for "holmium
sulfamate" turn up literally nothing.
<img src=http://i.imgur.com/gmSROtB.jpg width=800>
<img src=http://i.imgur.com/DGOTmIs.jpg width=800>
[Edited on 23.9.2015 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Nice ! Couldn't find anything on it, either. But I don't have that much
literature on f-Metals. Maybe in a very old or very large book.
Due to the amount of Copper Sulphate I recentely bought I started
to do some copper chemistry. The combined rests were collected in
a 1L Erlenmeyer Flask and today it had that color. And the cam really
picked it up correctely for once  The beautiful blue hue varies from the top to
the very bottom. From a dark blue to a very bright blue and then a The beautiful blue hue varies from the top to
the very bottom. From a dark blue to a very bright blue and then a
greenish blue. Really nice color, guess due to the Hydroxdie an Ammonia.
The rest was an attempt to make Copper(I)Bromide from Cu(II), Sodium Sulfite and KBr. I got that slightley yellow ( not really good on cam) compound
that now after a while turned green. So seemes to have worked but due to the contamination with Cu(II) I already put it to the waste.
Last there is an old experiment I wanted to do again, mixing a Cu(II) Salt with NaI which forms the instable Cu(II)Iodide that will form Cu(I) and
elemental Iodine. I added a bit of Heptane and let the layers seperate. Since it wasn't good on cam I got a bit of the Heptane layer into a Pipette,
so there is the purple layer with the Iodine dissolved on the top and a brown, now after washing it 3 times with Water white, cloudy solution.

[Edited on 24-9-2015 by fluorescence]
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
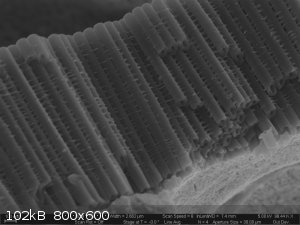
Titania Nanotubes (TNTs). Gotta love the acronyms people come up with for nano-structures.
These were anodically grown in an ethylene glycol electrolyte containing a small amount of ammonium flouride and water.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Here is some Bismuth Chemistry:
From the left to right:
Bismuth(III)Iodide, a dark compound that formes when Iodide is added to a Bismuth(III)-Solution.
Tetraiodobismutate, an orange coordination compound that formes if you continue to add Iodide. At the bottom are the Bismuth(III)Nitrate Crystals I
used as Bismuth Source, problem is that it's really insoluble. Luckily, traces of Bismuth are enough to from these colored compounds.
Bismuth-Chromate ? Dunno what that is. Added some Potassium Dichromate to a Bismuth(III) solution. Immedialtely yellow flakes appeared. Looks quite
cool but I can't really find anything on that compound.
Colin MacKenzie says it's Bismuth(III)Chromate in his work "One thousand processes in manufactures and experiments in chemistry"
But that book is from 1825 and so I doubt the analytical background a bit.
The Pigment Compendium talks about a Basic Dichromate that is formed when the Dichromate is added. Since I used some Dichromate instead of Chromate I
believe this compound is more acurate than the one before,
the formula here is given by Bi2O3 x 2 CrO3. I guess I had luck that I chose the Dichromate Bottle instead of the
Chromate bottle (which was just closer at that time xD ) since only that compound is described in the book.

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
And even more Bismuth Chemistry coming up, just the stuff I still had on my mind but I'll check literature for more.
So here is on the left a very famous compound, it's a Bismuth-Thiourea-Complex usually formed during the Bismuth-Slide. I dunno if that experiment is
still common in the lab but it's very good to find Bismuth in contaminated mixtures. What you prepare is a filter paper and you fold that so you get
like a little canal where a liquid can travel throught. Then you add your substance in the middle and the rest is stacked on top creating a little
pile, you add Sodium Fluoride ( to get away Aluminium and Iron ) then a Chloride ( to get away silver and mercury ) and Sodiumpotassium Tartrate
(Antimony and Tin ) and finally Thiourea. Then you add a bit of Nitric Acid and let it slide though the canal into the mixture. What's happening is
that all other Elements that could form a yellow compound with Thiourea will form a stable salt or coordination compound like the Iron Flouride and
thus create a yellow color ( if you ever did the experiment where you add Fluoride to an Iron-Thiocyante Complex you know how stable the colorless
Iron-Fluoride Complex is). And so only Bismuth will hopefully show a yellow color. I did that with just adding a bit of Thiourea and Nitric Acid to a
bit of Bismuth Nitrate solution.
On the right is the Dimethylglyoxime-Bismuth Compound formed, when those are added in Ethanol/Water and afterwards a bit of Ammonia is added. It will
take some time but after a while and if the conc. of Ammonia is high enough you'll get a yellow solution.

|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Long TNP crystals
take a look at the size of ths picric acid crystal garden i grew. these crystals are huge. the longest one is 4in plus.
This synthesis has become one of my favourites to perform recently.

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
What a beautiful purple color...if I only knew what it was.
That happens when you take the wrong metals...should have
taken Manganese but took Chromium accidentally when that formed.
Dunno. Should be some kind of K3[(CN)5NO]. At least it would have been it if I used manganese. There is that Cr(I)-Complex but
that one is supposed to be green, not purple and my Manganese one should be purple but is green....I have no clue what's going here.
But the Educt was highly saturated with Cyanide usually not attacked by Bases and yet the insoluble green to yellow Cyanide Salt changed in a second
to a soluble purple solution...too fast to be the Aqua-Ligand and if there is Cyanide in it I really doubt it anyway...but what is it xD.

|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Organic chemists be like "picturez of white substancez much".
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Had to do some Iron Chemistry for a Presentation. One of it was to make
Prussian Blue. That's all the wastes combined....

|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
Today I found a Uranium glass bowl in a second hand shop for $2 as well as some chemistry books, if I can find my UV light I will post a pic of it
fluorescing

[Edited on 4-10-2015 by NedsHead]
|
|
|
Dmishin
Harmless

Posts: 32
Registered: 5-10-2015
Member Is Offline
Mood: No Mood
|
|
Ferric alum. I made it from ferrous sulfate, baking soda, bleach, sulfuric acid and ammonia solution.
First precipitated FeCO3 by soda, oxidized it with bleach, washed sediment of Fe(III) oxides/hydroxides, then dissolved it in 43% acid, added
(NH4)2SO4 and re-crystallized.
Alas, I was unable to get clear crystals like I saw somewhere on this forum. Never expected such color from Fe(III) salt.
 
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Quote: Originally posted by Dmishin  | Ferric alum. I made it from ferrous sulfate, baking soda, bleach, sulfuric acid and ammonia solution.
First precipitated FeCO3 by soda, oxidized it with bleach, washed sediment of Fe(III) oxides/hydroxides, then dissolved it in 43% acid, added
(NH4)2SO4 and re-crystallized. Alas, I was unable to get clear crystals like I saw somewhere on this forum. Never expected such color from Fe(III)
salt. |
This is absolutely gorgeous! How long did that thing grow for? Looks like the size of a golf ball.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
That is a beautiful crystal.
Please post a step-by-step procedure so we can all make one.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by zts16  | Quote: Originally posted by The Volatile Chemist  | Interesting. I was slightly suprised to find barium had an insoluble citrate. This fact salvaged an attempt to make barium hydroxide (which didn't
precipitate well enough to collect). Like the bad chemist I am, I just threw some citric acid into solution and fitered the precipitate. So I at least
got one compound for my barium collecton. Which is starting to get some size 
|
You have a barium collection? Must be very white! 
Try making barium manganate if you haven't yet though. I've tried it a few times with partial success. It's supposed to be dark blue, but mine keeps
turning brown about a day after I wash and dry it. |
Yeah, I'm pretty white too.  I have Barium Potassium ferrocyanide crystals,
which are straw colored. Yes, mostly white, though, but I have made only about 7 compounds, it's hardly a collection. By the way, will nitrate oxidise
sulfite readily? I made some 'barium sulfite' from barium nitrate, but I realized later the sulfite could've just oxidized... I have Barium Potassium ferrocyanide crystals,
which are straw colored. Yes, mostly white, though, but I have made only about 7 compounds, it's hardly a collection. By the way, will nitrate oxidise
sulfite readily? I made some 'barium sulfite' from barium nitrate, but I realized later the sulfite could've just oxidized...
I don't have any manganate salts yet, haven't purchased any. I recently acquired a few more chemicals from the hardware and grocery stores nearby
(NaOH, for the first time, finally! (Though I couldn't get Ba(OH)2 to precipitate :/), also CaCl2 as pickling agent, anhydrous,
and Citric acid.) I'm trying to exhaust my lab of things I can make before getting any more reagents, so I probably won't try that till next summer.
But It's a good Idea, thanks. Hopefully I can get it to work.
Fluorescence, I'm not well educated on the 'nitroferrocyanide' (?) complex ion. Is it formed when a nitrate salt is introduced to a solution of
ferrocyanide or ferricyanide? I made some barium potassium ferrocyanide from barium nitrate and potassium ferrocyanide, but now I wonder if the
crystals precipitated contained this ion instead. The literature called for barium chloride, which I did not have.
|
|
|
Dmishin
Harmless

Posts: 32
Registered: 5-10-2015
Member Is Offline
Mood: No Mood
|
|
Thanks! Not that long: only 3 weeks, and I took measures to slow down growth (covered the beaker). This compound can grow really fast thanks to it
great solubility: 124g/100ml.
Nothing special, actually. I explained it briefly above. But, as you wish.
My goal was to use only compounds, available outside of specialized chemicals store. So, my preparation is surely not the easiest way to get this
salt.
Source compounds:
Ferrous sulfate FeSO4*7H2O, fertilizer grade
Baking soda NaHCO3, food grade
Bleach NaClO solution, 5-15% according to labeling.
Sulfuric acid 43%, electrolyte
Ammonia solution 10%, from a drug store (the most limited reagent for me).
Prepare (NH4)2SO4:
Accurately and quickly pour calculated amount of H2SO4 into ammonia solution while stirring constantly. With 10% ammonia and 43% acid, solution
becomes very hot (80 - 90 C), but not boiling. Beware boiling, burns, cracking glass and NH3 fumes while reaction is not yet complete.
Prepare Fe2(SO4)3
1. Dissolve FeSO4 in the minimal amount of hot water, add soda in small portions. A lot of CO2 is produced, beware excessive foaming. The sediment is
ferrous carbonates and hydroxides.
2. Without washing, oxidize the sediment with bleach. Add bleach in big portions (it is quite dilute). Sediment becomes brown, gas (CO2) is produced
(FeCO3 oxidizes to Fe(III) oxides/hydroxides and CO2 is left). The reaction is slow (hours), heat it mildly on water bath. After finishing reaction,
let it stay, decant the excess solution and add new portion of bleach. Stop, when decanted liquid starts smelling chlorine. The reaction is:
2Fe(II) + NaClO -> 2Fe(III) + NaCl
For me, it required 400% of calculated amount of bleach. Either my bleach was too dilute, or there were side reactions, consuming hypochlorite (ClO3
formation?).
3. Wash the sediment, remove the excess water, then add sulfuric acid (stoichiometric amount + small excess). It dissolves very badly: took 2 days.
Heating on hot water bath improves it. Filter the product.
You'll get dark brown solution of Fe(III) sulfate.
Prepare the double salt
Mix ammonium and ferric sulfates in equimolar amounts, let the solution evaporate (avoid strong heating, it causes hydrolysis). Ferric alum forms
almost colorless crystals. Collect them and utilize the last small portion of solution that contains excess chemicals and impurities.
Then grow the crystals in the regular way. My preparation is lengthy, but it avoids uncommon reagents, hazardous gases, need to boil down large
amounts of solutions and separate significant impurities.
[Edited on 7-10-2015 by Dmishin]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Very nice, good write-up.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
got a couple nice potassium trisoxalatoferrate(III) crystals. all it took was some patients and my previous solution yielded much better samples. I
noticed a few clear crystals in the mix, so reagent use wasn't dead on. but that doesn't bug me in light of the results  . the color is much more intense in person, but my phone was balancing it poorly
against the background of a white door. nice emerald green . the color is much more intense in person, but my phone was balancing it poorly
against the background of a white door. nice emerald green
  
and a zirconia disk I will use for a standoff while torching precious metals

|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
The Uranium glass bowl I found in a second hand shop for $2 under UV light

|
|
|
greenlight
National Hazard
   
Posts: 753
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Magnalium polumna firecracker at sunset:
[Edited on 24-10-2015 by greenlight]

Be good, otherwise be good at it 
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
NedsHeads, nice! The potassium trisoxalatoferrate(III) crystals are huge! We made some nice ones in AP chem, but the teacher made us use a mortar on
them....
|
|
|
Dmishin
Harmless

Posts: 32
Registered: 5-10-2015
Member Is Offline
Mood: No Mood
|
|
Monoclinic crystals of ammonium zinc sulfate hexahydrate. Belongs to the family of Tutton's salts. Shiny as brilliants, my photos can't show it fully.
(NH4)2Zn(SO4)2·6H2O
There is one trick to get very clear crystals: after preparing saturated solution, add baking soda (NaHCO3) to it, 0.5g of soda per 100g solution, and
stir. The reaction will start, producing some gas (CO2) and sediment (mostly ZnCO3). Let it stay for a day, then filter the solution and use it for
growing. This procedure drastically improves quality of crystals.
Authors of this procedure assumed that it precipitates impurities (Al and Fe3+), though I have some doubts. Maybe, crystal growth is
affected by lower pH, or by ions of Na.
 
[Edited on 25-10-2015 by Dmishin]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Those are crazy big... and I have a lot of zinc sulfate... Too bad I don't have a fume hood to quickly make some ammonium sulfate.
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 131
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
Potassium explosion on the top of water

|
|
|
Dmishin
Harmless

Posts: 32
Registered: 5-10-2015
Member Is Offline
Mood: No Mood
|
|
If you are going to try 2NH3+H2SO4, then it is not really needed, reaction finishes instantly, if acid is sufficient and reagents are mixed well.
However, if your reagents are concentrated (at least, more concentrated than 10% NH3 and 43% H2SO4, I used), mix could start boiling.
Ammonium sulfate can also be bought as fertilizer (haven't seen though), and of course, chemicals shops have it, in good purity.
If you have a lot of ZnSO4, and don't mind wasting half of Zn, using fertilizer ammonium nitrate (or chloride) is an option. Double Zn-NH4 sulfate is
much less soluble than its constituents. Mix concentrated, hot solutions of ZnSO4 and NH4NO3, and double salt will precipitate:
2ZnSO4 + 2NH4NO3 + 6H2O -> (NH4)2Zn(SO4)2*6H2O ↓ + Zn(NO3)2
(haven't tried exactly this reaction, but it must work)
[Edited on 26-10-2015 by Dmishin]
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
That is pretty cool. How did you capture that shot?
|
|
|
| Pages:
1
..
36
37
38
39
40
..
77 |