| Pages:
1
2
3
..
9 |
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Gold Recovery Computer Scrap Complete Process
Here is a video that I produced that uses chemistry to get gold from scrap computer circuit cards and then refine that gold to high purity, using only
household chemicals through the whole process.
I normally don't mess with computer scrap because of the low yields.
But producing this video was fun and it only took 24 hours from start to finish.
Any critical comments are welcome.
https://youtu.be/2sZUAprS5KI
Thank you
kadriver
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Fabulous !
Excellent video kadriver and a really great twist on the whole Gold refining chemisty approach.
You have made a Standard by which chemistry videos should be made.
Clear statement of the reagents, the quantities, and clear demonstration of the process.
I am sure i saw at least one of your videos on gold refining before.
Your style is unmistakeable.
[Edited on 13-9-2015 by aga]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
if you work out the figures you have lost money  . it cost you $70 (roughly and not
including chemicals) to get 1g Gold at $35 a gram. You might want to rethink your business model, maybe sell pcb trimmed fingers on ebay? It looks
profitable to sell them to people who want to refine them. You can sell them $35 of gold for $70!! . it cost you $70 (roughly and not
including chemicals) to get 1g Gold at $35 a gram. You might want to rethink your business model, maybe sell pcb trimmed fingers on ebay? It looks
profitable to sell them to people who want to refine them. You can sell them $35 of gold for $70!!
[Edited on 14-9-2015 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
wonderful! teaching means a lot to you even if it costs you some.i learned a lot.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
I have been watching your vids for a while now. thanks. I like the style and direct nature. I have done some cell phone and e-waste gold recycling
my self. but after all the hours of work, cost of reagents and torch fuels = its a learning experience kind of thing unless you find your scrap free
or really cheap.
LGA: but the gold doesn't have to be sold immediately, one can wait for a more favorable price to sell it. or sell it on ebay to would be investors
for an inflated price due to speculation and ebay fee's. cause aint no one gonna let ebay get 13% of the sale cost on gold at market value... they
over charge and get what's theirs + some usually.
[Edited on 14-9-2015 by violet sin]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by violet sin  | | but the gold doesn't have to be sold immediately, one can wait for a more favorable price to sell it. or sell it on ebay to would be investors for an
inflated price due to speculation and ebay fee's. cause aint no one gonna let ebay get 13% of the sale cost on gold at market value... they over
charge and get what's theirs + some usually. |
Pretty long wait for it too double in price? If its a learning thing why buy more than 500g??
[Edited on 14-9-2015 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
I surely did not crunch the numbers. I was just saying if it was off a bit, it could be accounted for. if you sell it off in small shot pieces for
element collections, that could be another way to inflate the price as well.
I did do a rough calculation on 500g = 1g @ 40$/g and noticed a big deficit. But didn't want to tie numbers into my statement here from my phone
without being *sure (science forum and all). But the miss's was using the laptop.. kinda makes the previous statement a poor substitute in lieu of
that amount of missing product. But would hold true if it were a few % off, b/c of a cheaper source.
[Edited on 14-9-2015 by violet sin]
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Quote: Originally posted by Little_Ghost_again  | if you work out the figures you have lost money  . it cost you $70 (roughly and not
including chemicals) to get 1g Gold at $35 a gram. You might want to rethink your business model, maybe sell pcb trimmed fingers on ebay? It looks
profitable to sell them to people who want to refine them. You can sell them $35 of gold for $70!! . it cost you $70 (roughly and not
including chemicals) to get 1g Gold at $35 a gram. You might want to rethink your business model, maybe sell pcb trimmed fingers on ebay? It looks
profitable to sell them to people who want to refine them. You can sell them $35 of gold for $70!!
[Edited on 14-9-2015 by Little_Ghost_again] |
Hello, please understand that I did not buy the fingers to make a profit, but rather to make a video. It would have taken me several years and
hundreds of dollars to buy enough scrap computers to accumulate 500 grams of trimmed fingers.
This is why I don't refine gold from electronic scrap; the yields are too low and you can't make a profit unless you get the scrap for little to
nothing.
I am doing some more experiments to try and shorten the time from start to finish. That is why I have all those extra fingers. This video was done in
just over 24 hours, start to finish.
I love tinkering and will be producing some more videos of these experiments. Making this video was much fun and I learned some new things that may be
incorporated into my refining techniques with other types of scrap.
Thank you for your comments.
kadriver
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Do you currently do runs of good filled or polishing sweeps? Any $ to be made there?
Another interesting way to go is the shor method with ammonium chloride, peroxide and electrolysis. No fumes.
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Quote: Originally posted by violet sin  | Do you currently do runs of good filled or polishing sweeps? Any $ to be made there?
Another interesting way to go is the shor method with ammonium chloride, peroxide and electrolysis. No fumes. |
Please see my other video entitled "Gold Refining Gold Filled Scrap Complete Process". Or just type in gold refining gold filled scrap in the search
block of Yahoo or Google. My video will come up on page one. My user name there is "sreetips"
I have several jeweler a that I work for, not retail jewelry stores, but actual jewelry repairmen. They give me bags of polish sweeps and filings to
refine for them. I charge 10% as my fee. If I get 75 grams of pure gold then I get 7.5 grams as my fee and they get 67.5 grams.
I don't give credit for the silver of platinum group metals. I get to keep those as well. It is tough to make a living doing this, that is why I do it
as a hobby!
I have never tried shor, don't know anything about it. I was trained on the goldrefiningforum dot com by Harold, lazersteve, GSP, Lou, Butcher and Oz
(just to name a few). All my experience is with wet chemical processes with nitric acid as a must.
But I am working on some experiments to eliminate the need for nitric acid. Nitric is expensive and not too easy to get.
Thank you for your comments,
kadriver
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
By the way, I am Terrible at chemistry. In this video I am adding bleach (sodium hypochlorite) to the gold foils that are in hydrochloric acid to
dissolve the foils. I think that the foils are dissolving from chlorine gas generated by mixing the bleach with the HCl.
Since sodium and chlorine are involved here I also think that there may be some table salt (sodium chloride) in with the resulting chloroauric acid.
Would someone be kind enough to explain what is happening in that beaker when I add the bleach to the gold foils in the HCl?
I need to sign up for some chemistry classes next semester. I'd like to know what's going on at the electron level when I do these reactions.
Thank you,
kadriver
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Shor process = off topic info.
US Patent 5009755 ( http://www.google.com/patents/US5009755 )
Ammonium chloride electrolytic leaching, activated with H2O2. worth a read. The bucket container with dividing ceramic chamber and electrode kit
costs like 500$. And you have to buy the chems from the company. Unless you read the patent above. There is also no power supply provided for your
investment.
[Edited on 14-9-2015 by violet sin]
From a gold refining forum... http://goldrefiningforum.com/phpBB3/index.php main page.
" . Chlorine is the active ingredient in dissolving the gold in the HCl-Cl reaction. " http://goldrefiningforum.com/~goldrefi/phpBB3/viewtopic.php?...
[Edited on 14-9-2015 by violet sin]
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Quote: Originally posted by violet sin  | Shoe process
US Patent 5009755 ( http://www.google.com/patents/US5009755 )
Ammonium chloride electrolytic leaching, activated with H2O2. worth a read. The bucket container with dividing ceramic chamber and electrode kit
costs like 500$. And you have to buy the chems from the company. Unless you read the patent above. There is also no power supply provided for your
investment.
[Edited on 14-9-2015 by violet sin] |
Nice. Thank you. Ill look at it carefully. Do you know anyone who has one? I'd like to get some feedback from any users who have operated this
apparatus.
kadriver
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Chlorine is dissolving the gold (I'm kind of sure that chlorine will dissolve just about any metal). But chlorine is not the only product in this
reaction. Im interested in knowing what other compounds are present as contaminants to my pure gold when I precipitate the metal with sodium meta
bisulfite.
And what kind of waste compounds will I be dealing with after I add the SMB. These are the kinds of questions I have since I have no chemistry
training.
Thank you,
kadriver
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I'm certainly no expert, however the starting material is a mix of several chemicals, some of which can end up in the solution.
The PCB will be multi-layer FR4 or similar (fibreglass).
The copper is bonded to that with something.
Then there's the Copper and the Gold.
There could be some tin, lead and whatever the solder resist mask is made of etc etc.
Not sure that Chlorine is reacting with the gold directly.
More likely it is a multi-step reaction, with the gold being complexed somehow and then further reacted to end up with chloroauric acid.
Edit:
Oh ! I see you've already tried it, and Chlorine + Hydrochloric acid can dissolve Gold.
Wiki says this reaction happens :-
2 Au + 3 Cl2 + 2 HCl => 2 HAuCl4
https://en.wikipedia.org/wiki/Chloroauric_acid
If you're looking to maximise the process/profits then one of the first things to learn in chemistry is stoichimetry, which lets you work out the
exact amounts of each chemical you need for a reaction.
[Edited on 14-9-2015 by aga]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hi kadriver.
The full picture of what's going on is a little more complex. The production of chlorine gas is, in fact, an unwanted side reaction in this system and
could/should potentially be eliminated or at least minimised.
To understand what is going on in the solution, we need to consult the Pourbaix diagrams for gold in chloride media (1) as well as that of chlorine in
water (2). Apologies in advance for lazy referencing, I'm battling a nasty flu at the moment.
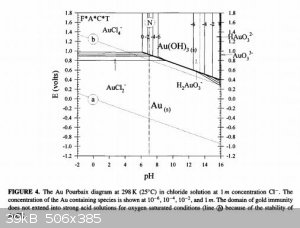 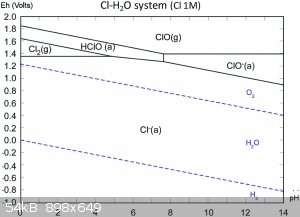
Firstly, take note of the AuCl4- region on the first diagram. It's rather large and goes up even to quite high pH, so you don't need extremely acidic
conditions!
Secondly, on the second diagram, note that in acidic media, hypochlorites form hypochlorous acid, a very powerful oxidant indeed, even more so than
chlorine (see how it's region lies at a higher potential than the chlorine region on the left?).
So from this diagram you can conclude that the oxidant is probably hypochlorous acid and that hypochlorous acid can decompose to chlorine gas if it
doesn't have anything else to oxidise.
Now here's the cool bit, if you're between pH 5-6 in a 1M Cl- solution, you can't form chlorine gas, but can form AuCl4- just fine, even up to very
high gold concentrations (1M), which you're far from anyhow.
So I suggest if you want to experiment with an improvement, work in a 1M sodium chloride solution (table salt) which is buffered to pH 5. That way you
are not wasting acid or bleach, creating less and less hazardous waste and avoiding the formation of toxic chlorine gas! If you were wondering, the
gold in solution would then be in the form of a solution of sodium tetrachloroaurate (III).
Please be careful when selecting a buffer to use. It should not contain ammonia! One possibility is a sodium acetate-acetic acid buffer, since it can
be prepared from vinegar and lye, all OTC and cheap!
From reference (3), mixing 70ml 0.2M sodium acetate solution with 30ml 0.2M acetic acid will give a buffer at pH 5. Note 5% commercial vinegar is
about 0.8M solution of acetic acid, so you will need to dilute. Don't forget you also need to add sodium chloride to prepare a 1M NaCl solution in
that buffer!!!
You could make this in advance and large quantities and potentially have a very cheap and benign media to do your reaction in!
Be careful to add you bleach slowly and incrementally so as not to overshoot too much as you might precipitate gold hydroxide, although you would need
a very large overshoot to do this as the solution is buffered. Just add enough to dissolve all the bits, then stop.
For now this is all theoretical, but I trust you might enjoy the experimentation and see the merits in this approach.
References:
Obtained online from:
(1) http://www.semos.dk/Per/41653/download/Pourbaix_multielement...
(2) http://pubs.rsc.org/en/content/articlelanding/2015/ja/c4ja00... whose image obtained online from Google image search with keywords "chlorine
pourbaix".
(3) http://www.sigmaaldrich.com/life-science/core-bioreagents/bi...
[Edited on 14-9-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Or even simpler, just add enough salt to vinegar to make a 1M sodium chloride solution and that would be good enough, even if it's more acidic. The
addition of bleach will result in a buffered system anyway in this case, since bleach is alkaline. With a bit of luck, the resulting solution is not
acidic enough to allow the formation of chlorine gas.
Finally, since you probably want to economise on reagents as much as possible, you may want to calculate how much reagents to use and allow for say
50% excess.
So for example, the half reactions for gold dissolving in chloride is:
2Au + 8Cl- => 2AuCl4- + 6e-
and for hypochlorous acid as oxidant:
3HClO + 3H+ +6e- => 3Cl- + 3H2O
So the overall reaction cancelling out is:
2Au + 3HClO + 5Cl- + 3H+ =>2AuCl4- + 3H2O
From an estimated 1g gold, you can back-calculate the amount of sodium hypochlorite solution needed at the concentration you buy it. With some fancy
chemistry calculation, you can also calculate the amount of acetic acid you would need that would result in a pH of 5-6 when all is said and done,
though this is probably easier to determine experimentally. Don't forget to allow for some excess.
Similarly, you can also consider the pourbaix diagrams of your base metals and construct better and more economical processes for their dissolution.
There may even be papers published about this. I've seen such a study taken for the recovery of tin from tin plate waste (cans etc.).
Ideally, you would want to use just air as your oxidant and not peroxide to reduce costs and you already using it to agitate your system!
[Edited on 14-9-2015 by deltaH]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
That's a pretty full answer deltaH !
Going to give this a try right now.
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
This is great info. I can see how a knowledge of chemistry would be so helpful in my experimentation. The explanations given above by aga and deltaH
are exactly the kind of information I was after. Even though I didn't quite understand it all, it has made it quite clear that I must begin a study of
chemistry to be able to do the kind of research I want to do.
I have dabbled with some of the platinum group metals. I have gotten about four grams of palladium from some previous experiments. But there is much
more chemistry involved. Compared to the platinum group metals, gold and silver are "like following a cookie recipe".
Thank you for those replys, very helpful indeed!
kadriver
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks, but take note, I'm under the affluence if vast quantities of codein phosphate, so I might just be missing some crucial aspect.
Some more blah blah:
If you rewrite the overall equation with regards to what we actually are using, i.e. sodium hypochlorite.
2Au + 3NaClO + 5NaCl + 6CH3COOH =>2NaAuCl4(aq) + 3H2O + 6NaCH3COO(aq)
Hopefully that's balanced. Someone, please check.
Now we can work out how much bleach to add and also know how much sodium acetate will form.
From the amount of sodium acetate that forms and this:
| Quote: | | mixing 70ml 0.2M sodium acetate solution with 30ml 0.2M acetic acid will give a buffer at pH 5 |
One can work how much extra acetic acid you need and volume of water to end up with the pH 5 buffer ignoring the sodium tetrachloroaurate. Don't
forget you also make some water in that reaction and also remember you need six moles of acetic acid per 2 moles of gold to generate that acetate in
the first place. I think this calls for the power of grey skull (or EXCEL and solver).
I'm too sick to do this now, but with this roadmap, I'm sure others can succeed.
NB: Pourbaix diagrams show the speciation of ions based on thermodynamics, they say nothing about kinetics (speed of reaction), just that given enough
time, when things equilibrate, that's what you end up with. So that said, I would expect this reaction possibly to be slower than what was observed
before, but since you can work out exactly the amounts to dose even at once, you just let it stand for a few hours if it's too slow.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's been minutes now, and nothing much happening ...
Edit:
If you're totally new to all the workings-out, here's a couple of brief explanations about Balancing equations and working out Moles and Molarity :-
Attachment: Balancing Equations.pdf (44kB)
This file has been downloaded 687 times
Attachment: Moles and Molarity.pdf (61kB)
This file has been downloaded 676 times
Appologies if they're too simplistic.
[Edited on 14-9-2015 by aga]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Process for recovering metals, in particular precious metals, from electronic scrap
United States Patent Application 20040179985
Attachment: US20040179985A1.pdf (80kB)
This file has been downloaded 782 times
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Okay, dosed up, feeling a little better, can attempt some number, but take it with a big pinch of salt (excuse the pun).
So let's work on a basis of 1g Au
Moles Au = 1g / (197g/mol) = 5.076mmol.
Moles of NaOCl required = 5.076mmol/2*3 = 7.614mmol.
Assuming bleach is 3.5% m/v sodium hypochlorite, that means we need (7.614mmol)/1000*(74.44g/mol)/(0.035g NaOCl/ml bleach) = 16.19ml bleach.
To start with, just for the equation, the amount of acetic acid we need is (5.076mmol)/2*6 = 15.228mmol
Assuming vinegar to be 5% acetic acid m/v, that means we need (15.228mmol)/1000*(60g/mol)/0.05g acetic acid/ml vinegar) = 18.27ml vinegar (not
counting the extra we will need to buffer with).
Now to work out the requirements to buffer this sucker.
Previously it was stated that for 70ml of a 0.2M sodium acetate solution...
First lets work out how many moles that is: 70/1000*0.2mol = 0.014mol or 14mmol of sodium acetate.
But how much sodium acetate did we actually make from our 1g gold: 15.228mmol
So using some ratio and proportion, we need: 15.228mmol/14mmol*70ml = 76.14ml to generate the portion of 0.2M sodium acetate solution.
For the recipe for the buffer, the 70ml 0.2M sodium acetate solution was mixed with 30ml 0.2M acetic acid, so using ratio and proportion again:
Amount of 0.2M acetic acid required: 15.228/14*30ml = 32.63ml 0.2M acetic acid.
But how much vinegar would yield that amount of acetic acid? 32.63ml/1000ml*0.2mol/l = 6.526mmol
That's 6.526mmol/1000*(60.5g/mol)/(0.05g acetic acid/ml vinegar) = 7.89ml vinegar
In addition to the amount required for the equation.
So the total amount of vinegar required is 18.27ml + 7.89ml = 26.16ml
Now finally we need to work out how much water to add to dilute this system.
From the buffer recipe, we want a total amount of water of 15.228/14*100ml, assuming the ions don't contribute to volume.
That's 108.77ml water. But the reaction makes some water and the vinegar and bleach 'bring' some water, so we need to subtract those.
Reaction water = 5.076mmol/2*3/1000 * 18.02g/mol= 0.137ml (neglegible)
Water from acetic acid = 26.16ml (assuming the acetic acid doesn't contribute significant volume... it does slightly because it's not fully ionised,
but the conc. is very low so probably not much) = 26.16ml
Water from bleach = 16.19ml (again assuming the ions have no volume) = 16.19ml
So water needed to be added = 108.77ml - 0.137ml - 26.16ml - 16.19ml = 66.28ml
Amount of NaCl required for 1M solution is 108.77ml total water (and assumed volume) /1000ml * 1M * (58.44g/mol) = 6.36g
SUMMARY:
To dissolve 1g gold flakes (rounding number up):
Dilute 27ml 5% m/v vinegar with 66ml water to which you add your gold.
Then add 17ml 3.5% m/v bleach.
Stir and wait...
EDIT: and 7g NaCl of course!!!   
[Edited on 14-9-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Aga, what gold are you using, the same type of flakes or a piece of gold? This is a surface reaction, so these ultrathin flakes would dissolve far
more rapidly than bulk gold. For the flakes, the gold need only 'corrode' by some microns in depth to be gone.
Another possible problem is that maybe the bleach is reacting with the vinegar. If so, we should change to a more inert weak acid.
Finally, maybe the amounts matter, try using the one I worked out earlier, to repeat (note I forgot the NaCl earlier, now corrected):
1g gold flakes
27ml 5% m/v acetic acid (white spirit vinegar)
66ml water
17ml 3.5% m/v sodium hypochlorite (household bleach)
7g NaCl (table salt)
[Edited on 14-9-2015 by deltaH]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Using only household chemicals, and your household fume hood and household acetylene torch  Not quite so easy! Not quite so easy!
A fume hood is definitely required for this. You've got tons of HCl, chlorine, and sulfur dioxide fumes throughout the process. I wouldn't be
comfortable doing this outside, even. It'd be impossible to work next to! Very cool video though, I enjoyed watching it. If I ever get around to
making my fume hood, this is something I'd like to try out.
|
|
|
| Pages:
1
2
3
..
9 |