| Pages:
1
..
34
35
36
37
38
..
77 |
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
most unexpected result j_sum. maybe different solvents will prove to make different crystals
Beginning construction of periodic table display
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
So, I heard you like pretty pictures 
https://hobbychemistry.wordpress.com/2015/08/18/returning/
PS: I was intending to post the photos here, but there are too many to resize, so I'll just direct you to the post with all of them.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Very nice! What is the first compound?
|
|
|
violet sin
International Hazard
    
Posts: 1483
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
found a nice book at the thrift store today  only $2.50 for 159pg's of decent
reading, I can't complain. I have found a few other great books there randomly from time to time. only $2.50 for 159pg's of decent
reading, I can't complain. I have found a few other great books there randomly from time to time.
 
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Online
|
|
j_sum1
nice sulfur crystals !
how long did they take to grow ?
[Edited on 22-8-2015 by Sulaiman]
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Just as long as it took for the xylene to cool.
Not very long at all.
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Quote: Originally posted by violet sin  | found a nice book at the thrift store today  only $2.50 for 159pg's of decent
reading, I can't complain. I have found a few other great books there randomly from time to time. only $2.50 for 159pg's of decent
reading, I can't complain. I have found a few other great books there randomly from time to time.
|
Nice, that looks very useful! (although I'm sure the people at the store would not understand)
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Time lapse would be cool for the growing of the sulfur.
Fine book! The only specialty book I've found at a half priced books of interest was a book having something to do with fields and molecules, I saw it
a few years ago and didn't know what it was. It seems like it had to do with meshes (like in engineering programs) and the fields of electrons or
charge that computer libraries like openbabel can calculate. But it was a while ago.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by violet sin  | found a nice book at the thrift store today  only $2.50 for 159pg's of decent
reading, I can't complain. I have found a few other great books there randomly from time to time. only $2.50 for 159pg's of decent
reading, I can't complain. I have found a few other great books there randomly from time to time.
|
Beware! Such buying can become addictive and compulsive.
Once started, you may find hard to stop 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I might look into that.
Here's the latest effort -- silver crystals via displacement reaction. Nothing spectacular but a bit of a classic. I was pleased with the way that
they look.

|
|
|
violet sin
International Hazard
    
Posts: 1483
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
first batch of crystals after cooling, then after filtered
 
second batch of crystals from remaining sol, and cool pattern left from a thin film drying
 
Potassium ferrioxalate I finally got around to crystallizing, in the third pic you can see what happened when I let it cool slowly while trying for
larger crystals. from the second wash after filtering. left in the pan on the hotplate with an aluminum lid for a slow cool. I got larger shapes,
of small crystals...
also, notice to the left of the container bottom of first image, you can see a decidedly darker green spot. apple green to my eyes. this spot was
set apart just lower left of center of the second image. I haven't had time to investigate yet, but it kinda looked like a larger crystal had formed
in the mossy aggregate of small crystals.
while watching the solution cool, you could see larger thin sheets materialize rapidly. they looked to be kinda like a square ripped diagonally in a
stair step pattern.
I often end up using 1$ coffee maker carafes for their large surface contact and cheap nature. gotta love the thrift store.
the first batch didn't turn out well, apparently hadn't read the part about sunlight and while in solution... bright 103'F day had me confused for a
bit, but reading fixed that. pH paper, and the basement did the rest.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
New Life begins ...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Beautifull babies...
May I ask: seeds or spores of what?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Starcruiser
Harmless

Posts: 18
Registered: 11-7-2015
Location: Orion Arm/Milky Way
Member Is Offline
Mood: No Mood
|
|
Potassium nitrate crystals
Potassium nitrate crystals (macro photography)
    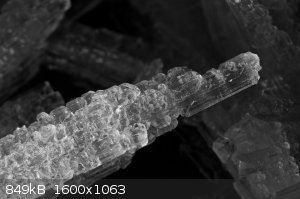 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
3 of Ox Heart tomato, one of basil (the blurry one).
70 hours from starting to try to germinate them, and not a drop of GA-3 in sight 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nice crystals Starcruiser.
They look great in black and white.
People forget that we see in B/W much better than in colour.
|
|
|
Starcruiser
Harmless

Posts: 18
Registered: 11-7-2015
Location: Orion Arm/Milky Way
Member Is Offline
Mood: No Mood
|
|
Thank you, Aga.
Some more KNO3:
    
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I can see this is a Fetish.
Stop before you go blind.
Edit :
Has anyone tried making a saturated solution, then dilute it just a tiny bit, then slowly dripped it from a height to make a stalagmite ?
It might work with KNO3, sodium acetate sounds easier.
[Edited on 25-8-2015 by aga]
|
|
|
Starcruiser
Harmless

Posts: 18
Registered: 11-7-2015
Location: Orion Arm/Milky Way
Member Is Offline
Mood: No Mood
|
|
It is something I wanted to do for a long time.
Good advice, though... will keep that in mind at least `till my next batch of nice crystals 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. The Race is On.
aga vs Starcruiser in the Stalagmite Challenge !
(dac 5.38)
3 days to produce a stalagmite of any substance of your choice, provided that it starts as a Flat plate and a solvated liquid.
3, 2, 1, Go !
I'm thinking sodium acetate and calculating the evaporation over 1 metre.
Splashes ! Damn. Need a Tall bucket or something.
|
|
|
Camroc37
Hazard to Others
  
Posts: 101
Registered: 4-8-2015
Location: USA
Member Is Offline
Mood: Must...Nitrate...EVERYTHING!
|
|
Hey, whats up?
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Please use the U2U message system if you wish to converse with others.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
I tried that sulfur crystalls with Benzene today.
Heated about 5 ml of Benzene with 2 Spatulas of Sulfur
to it's boiling point, had it boil for about 5 minutes and
then wrapped aluminium foil around the test tube.
That's what I got.

Quite nice chunks for the fact that I started with a powder
it looks quite cool 
[Edited on 28-8-2015 by fluorescence]
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by fluorescence  | I tried that sulfur crystalls with Benzene today.
Heated about 5 ml of Benzene with 2 Spatulas of Sulfur
to it's boiling point, had it boil for about 5 minutes and
then wrapped aluminium foil around the test tube.
That's what I got.
Quite nice chunks for the fact that I started with a powder
it looks quite cool 
[Edited on 28-8-2015 by fluorescence] |
try to go with more solvent in a 50 cc flask, add a small piece of random rock as a seed rock (  ) and cool it down slowly (I used dewar´s botttle) ) and cool it down slowly (I used dewar´s botttle)
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Thin, plate like crystals of 4-amino 2,6-dinitrophenol, resembling a pure gold pigment powder when dry. Recrystallized from ethanol/water, the wet,
thin plates orient themselves as one reflective gold mirror, truly one of the most beautiful compounds I made so far. 

|
|
|
| Pages:
1
..
34
35
36
37
38
..
77 |