| Pages:
1
2
3
4 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Here is a useful book
This book should be put in the forum library but I am not sure how to upload it to that destination, so at least temporarily I have placed the pdf
file here for the book
Aqueous Solution And The Phase Diagram
http://sciencemadness.org/scipics/Aqueous%20Solution%20And%2...
This book helps understanding the physical chemistry that comes into consideration with solutions, presented as an introductory level sort of text.
Really for our interests with regards to nitrates, much more comprehensive studies and charts of highly concentrated mixed nitrate solutions and
substantially anhydrous or low hydration eutectic melts is needed, but this particular book is a start and could be a helpful guide into understanding
and charting ones own experiments if published references are not available for
nitrate salt systems of interest.
Here is a page that offers a free program having a Microsoft excel template for triangular diagrams which may be useful not only for nitrate solution
calculations but possibly may be adaptable for workups of nitration diagrams. I have not tried this program so I can offer nothing further about it at
this time. On second glance it appears the ions of particular interest are not included with the free version of this software. A fully functioning
program covering the nitrates
of NH4+ Na+ K+ Mg++ Ca++ Zn++ Al+++ Cu++ Mn++ ect.
for systems up to quinary level would be more like what we would find interesting.
http://www.phasediagram.dk/index.htm
@ Anders Hoveland it appears Kaj Thomsen is in your neighborhood, so maybe you can facilitate getting us all a free trial program for the nitrates and
mixed nitrates 
[Edited on 13-9-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
85C 1% H2O nitrate brine 70.7% NH4NO3 28.3% NaNO3
Quote: Originally posted by Rosco Bodine  | Quote: Originally posted by quicksilver  | | Quote: | Originally posted by Rosco Bodine
The use of nitrates as concentrated solutions does simplify addition of the nitrate to the sulfuric acid solution of
the sulfonated organic material to be nitrated . I have mentioned that using
a solution of two or more different nitrates
can make possible an even more concentrated solution having less water content which would dilute the nitration mixture . Such solutions of mixed
nitrates have been developed to provide a liquid oxidizer phase having low water content for use in manufacture of emulsion explosives . Some of
these solutions are
essentially a eutectic salts mixture which
also exhibit a enhanced cosolubility in the presence of a small amount of water , similarly as they have a much lowered melting point even in the
absence of water altogether.
I know I have seen other compositions mentioned and I will share any others I find . |
I was unawair of the "mixed-nitrates" concept...and PLEASE; if you do find material related I would deeply apprieciate seeing it!
- I too use the PATR but find that patents are making much more impact in finding answers, new proceedures, & use of my time. Some of the most
interesting stuff I have found have come from US patents thus far.
As I have the same trouble accessing UK patents, as you have noted yourself, that site is a real pain. But the older, valuable patents (where they
were nitrating everything under the sun) and the older techniques, seem to originate in the UK. |
Here are some reciprocal solubility charts for a couple of the binary systems of mixed nitrates which indicate the co-solubility enhancement which
occurs at certain temperatures anyway for certain unique proportions of salts in mixture in water solution. There are definitely other binary and
tertiary and possibly quaternary systems where a peculiar increase in water solubility occurs for certain specific proportions of particular nitrates
in mixture. Calcium nitrate and ammonium nitrate exhibit reciprocal solubility, and I think it is also true for magnesium nitrate with other
nitrates which would be very interesting. Where the indexing of such reciprocal solubility data is to be found I do not know.
To anyone who may be helpful with reciprocal solubility data references for nitrates, please add to these references. |
More on reciprocal solubility of the sort that is interesting in regards to potential usefulness in nitrations, where the nitrate may be added as
liquid from a heated addition funnel. See the attached patent US3734709 on the last page, last paragraph just before the claims, where a concentrated
mixed brine of ammonium nitrate and sodium nitrate is specified liquid at 85C which contains only 1% H2O
and has a solid nitrates content of 99% ...which is the kind of very low H2O content mixed nitrate system being sought.
There are probably others, but this looks like probably a very good combination.
If the patent's reported composition is accurate, the ratios would correspond on a dry basis of a total 100 parts for the nitrates, 28.55 parts NaNO3
71.45 parts NH4NO3
The dramatic effect of lowering the liquid temperature caused by the 1% H2O can be seen by considering the eutectic point for the anhydrous salts
mixture is 35C higher. See attached articles which describe the eutectic for the anhydrous salts mixture. Also attached is an article describing the
solubility in H2O for NH4NO3 alone.
Attachment: US3734709_AMMONIUM_SODIUM_NITRATE.pdf (110kB)
This file has been downloaded 918 times
Attachment: The Properties of Ammonium Nitrate. Part III. Ammonium Nitrate and Sodiurn Nitrate.pdf (430kB)
This file has been downloaded 1208 times
Attachment: The System Ammonium Nitrate-Sodium Nitrate.pdf (543kB)
This file has been downloaded 1318 times
Attachment: The properties of ammonium nitrate. Part II. Ammonium nitrate and water.pdf (309kB)
This file has been downloaded 1848 times
[Edited on 15-9-2011 by Rosco Bodine]
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Picric acid turned black?
I'm familiar with the synthesis of picric acid(trinitrophenol) and have successfully made it twice using aspirin, concentrated sulfuric acid, and
KNO3. The most recent synthesis I conducted was in a much larger volume than the previous two. I dissolved 42g aspirin in 200ml of ~96% sulfuric acid,
let it sit at about 90 degrees celsius until all aspirin was dissolved and a black colour was achieved. Next I slowly added 80g KNO3 in parts to the
mix, carefully keeping the temperature low enough so as to evolve minimal nitrogen dioxide fumes. When this was done, and the solution was rapidly
cooled in an ice bath, I got, as usual, lots of crude picric acid and a lot of yellow solution. Everything seemed fine. But when I filtered it out and
added the crude TNP to boiling water in an attempt to purify and remove the excess acids, something funny happened. Some of the impurities came out as
a red foam, which is normal in my experience, but after a while the entire contents of the beaker turned very dark yellow/black. I'm afraid I may have
ruined my batch, but can anyone tell me for sure, and if so, how not to do it again? Sorry for the long post; I ramble.
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Did you purify the ASA out of the pills ?
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
Bert
|
Threads Merged
30-4-2014 at 10:00 |
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
In fact I did; I keep a large amount of it already purified as a reagent. In the end, after my first couple of ideas didn't work, I ended up adding
some sodium bicarbonate to the solution, which miraculously seemed to clarify it back to a more acceptable red color, and after
filtering/boiling/recrystallizing, ended up with a decent amount of clean picric acid.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Just an observation that I wanted to share, not really of added value to this thread.
Just recrystallized a batch of PA for picramic synthesis, Normally I let the water solution cool and don't give it a closer look untill the next day
or so. I never noticed how beautifull this process is. Upon a closer look the feather like crystals beginning to grow also introduced pronounced
turbidity (dont know if this is the right word), probably due to the energy of their formation. Almost looked like the solution was on a hotplate, or
mixing ethanol and water. Reminded me of a black smoker. It is somewhat visible in the top right of the photo. :-)
Maybe I just inhaled too much NO2... 

[Edited on 14-6-2014 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Things that have been seen cannot be unseen  On page 1 of this thread On page 1 of this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=4457&a...
Quote: Originally posted by Rosco Bodine  |
In near saturated solutions very near the boiling point , I have observed nascent crystals of picric acid first appearing as merely a pinpoint of
reflected light grow to flat rectangular plates with pointed ends attain 10 mm length in 5 minutes . In bright light , a spiraling ribbon " mirage
effect " in the liquid off the pointed ends of the crystals can be observed as the telltale sign of current in the liquid dragged along by the
depositing molecules which build the crystal larger at a barely perceptible visible rate . The rate slows down drastically at lower temperatures and
you cannot actually watch an individual crystal grow in length .
|
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
How could I think this had escaped the attention of the SMDB picric acid expert himself?  Regrettably however, I must admit that (despite the usually highly interesting and detailed content of mr. Bodines posts), I have not read
all 5146 of them. Regrettably however, I must admit that (despite the usually highly interesting and detailed content of mr. Bodines posts), I have not read
all 5146 of them.
Anyway, I gave the ascorbic acid reduction a shot to picramic (small scale). Characteristic red colour developed after prolonged boiling Sodium PA
with slight excess of sodium ascorbate, the colour very much resembles that of (poly)sulfide reduction, a really dark red, almost black. Now the funny
thing, whereas immidate precipitation is evident with (poly)sulfide reduction, nothing crystallizized after the ascorbic acid reduction. Not even
after 2 days at 4 deg C. Volume was boiled to 1/3 of original, 2 days 4 deg C, nothing. Then added 10% HCl, no picramic acid precipitation. What is
going on here? Is the SPA reduced further to more produce more soluble products or does picramic acid under some condition refuse to crystallize from
super saturated solultions? Solubility is like 1-2 g/l at RT IIRC. Opinions/suggestions?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It is essential that the stoichiometry be based upon reduction involving the sodium salt of picric acid, because that is actually what is done. The
reduction is pH dependent.
The reduction of sodium picrate to sodium picramate using sodium ascorbate I haven't done so I can't share any comparison observation. But I would
expect the reduction to occur quickly at well below boiling, like at 60C should be fine.
At boiling, I am not sure how stable the picramic acid would be and it could be decomposed by an unfavorable pH. If the amount of reducing agent
isn't limited, the reduction could also go further and reduce more than the 1 desired nitro group so you could end up with diamino or even
triaminophenol instead of the desired picramic acid. The solubility of the sodium salt of picramic acid is not very great and the solubility of free
picramic acid from acidification is extremely small. The sodium picramate is however an intense dark maroon red "oxblood" color dye even appearing
almost black in concentrated solution, but should color fade to almost clear very pale yellow tint on acidification, precipitating the nearly
insoluble free picramic acid, which should be a dull brownish color, IIRC. You sure wouldn't need to concentrate the solution to isolate any free
picramic acid because it is virtually insoluble the solubility is so low.
[Edited on 20-6-2014 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Reaction conditions and quantities were initially used from the attached article where they indeed mention preferential reduction of only one nitro
group. It was however on a less than a gram scale and pH was not monitored, so afr from ideal indeed. :-) The reduction, as judged by the rate of
colour change is surprisingly slow even at 70-80 degrees, since I also had other things to do that day, I heat it to the boiling point to speed things
up. The article (if you can call it that) mentioned however that the redcution products from dinitrobenzene reduction using AA could not be isolated.
Could it be that the dehydroascorbate from the reduction acts as a solubility modifer? It is unclear where they refer to exactly. Might be a good test
to see whether isopropanol would be able to precipiate the sodium dehydroascorbate while keeping Sodium picramate in solution, maybe extraction?
Suitable solvent?
Attachment: Reduction of nitrobenzenes with ascorbic acid.pdf (155kB)
This file has been downloaded 1176 times
[Edited on 21-6-2014 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The use of ferrous sulfate is mentioned so it is entirely possible the actual reduction is accomplished by the ferrous hydroxide regenerated from the
ferric by the ascorbate. Ascorbic acid is reported to reduce ferric sulfate to ferrous sulfate and similar reductions are reported where use of
ferrous compounds is reported in schemes where there is regeneration of the spent ferric byproduct with it reduced back to the ferrous active reducing
agent by various reducing agents. Iron or perhaps other metal filings, reducing sugars, or sulfides and ascorbate can all be used in such schemes
where iron salts are catalytic and regenerated. This may be a key aspect for the reaction, but I'm not certain.
I think there is a similar catalytic reduction possible likewise using manganese and copper as regenerable catalytic reducing agents. What is
operative for such schemes is I think the selectivity of the main reducing agent is better for rapid reaction regenerating the catalyst which reacts
more readily with the nitro compound. It could be that the hybrid catalytic reduction scheme there is necessary for the reduction to proceed at
moderate temperature. There is a dedicated thread for picramic acid where various processes are referenced.
The yield for the sodium sulfide reduction is very high and is reportedly high yield for some of the catalytic schemes also. If one reduction scheme
fails to work well then there are several alternative schemes possible.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Nitrates Eutectic Mixtures
Some mixtures found listed on use net 20 years ago
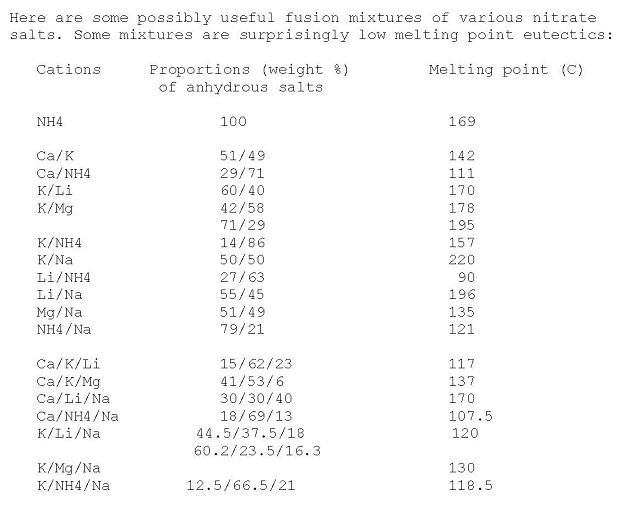
Edit: attached is the original source posting. I have edited and added some of the missing data in the K/Li/Na entry, gotten recently from other
sources
Attachment: eutectic nitrate mixtures usenet.pdf (9kB)
This file has been downloaded 742 times
[Edited on 10-9-2014 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Magnesium Nitrate Mg(NO3)2 dehydration related
More information provided here concerning dehydration properties of Magnesium Nitrate which is useful alone or in mixtures for oxidizer or nitration
applications.
References attached relate to hydration states of Magnesium Nitrate and describe thermodynamic studies including vacuum dehydration of the hexahydrate
to the dihydrate which is reported surprisingly to occur faster at a lower temperature of 40 - 45C range than at only moderately higher temperatures.
What would occur at a much greater higher temperature as for increasing the rate of dehydration under vacuum is not described.
The magnesium nitrate - water binary system
From the following page
http://www.phasediagram.dk/binary/magnesium_nitrate.htm

The magnesium nitrate - water binary system exhibits a particular solubility behavior encountered in many aqueous salt systems: multiple solubilities
at the same temperature. Between 50 and 90°C the solubility of magnesium nitrate can be one of three values, as it appears from the phase diagram at
the right. The figure also shows that the Extended UNIQUAC model is able to reproduce this type of solubility behavior quite accurately. Three
eutectic points and one peritectic point are found in this binary system.
The eutectic point where ice and magnesium nitrate nona-hydrate precipitate simultaneously is at -27°C and 33 % Mg(NO3)2. This is the cryohydratic
point. A peritectic point appears at -21°C and 36 % Mg(NO3)2 marking the transition between the nona-hydrate and the hexa-hydrate of magnesium
nitrate.
The hexa-hydrate and the di-hydrate of magnesium nitrate form a simple eutectic system with a eutectic point at 50°C and 67 % Mg(NO3)2. Finally
magnesium nitrate di-hydrate and anhydrous magnesium nitrate form a simple eutectic system with an eutectic point at 129°C and 82 % Mg(NO3)2.
The fact that the transition at 129°C is a eutectic rather than a peritectic point has been documented in a number of experiments as for example the
measurements carried out by Ewing, W. W., Brandner, J. D., Slichter, C. B., Griesinger, W. K. "The temperature-composition relations of the binary
system magnesium nitrate-water", J. Am. Chem. Soc. 55(1933)4822-24.
(JACS article and related articles attached)
Attachment: Temperature Composition Relations of Binary System Magnesium Nitrate - Water.pdf (154kB)
This file has been downloaded 683 times
Attachment: THERMAL BEHAVIOUR OF Mg(NO3)2 6H20.pdf (444kB)
This file has been downloaded 964 times
Attachment: Vacuum dehydration Magnesium Nitrate.pdf (564kB)
This file has been downloaded 1355 times
The fourth file attached describes a dehydration of Mg(NO3)2
Thermodynamic properties of potassium nitrate - magnesium nitrate compound [2KNO3 - Mg(NO3)2]
Attachment: Thermodynamic properties of potassium nitrate - magnesium nitrate compound [2KNO3 - Mg(NO3)2].pdf (321kB)
This file has been downloaded 1596 times
[Edited on 26-3-2015 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
"nitrous gases" useful for nitrations
In a previous post the subject of nitrosylsulfuric acid was brought up as being an observed component of the nitration mixture producing picric acid.
http://www.sciencemadness.org/talk/viewthread.php?tid=4457&a...
There has been passing interest in this observation because it is not really known for certain if the nitrosylsulfuric acid is entirely just a
byproduct accumulating in the nitration mixture or if it is actually an essential intermediate component which participates in the nitration and may
be the actual nitrating agent derived in situ from the heated nitric and sulfuric acid mixture. There have been reported high yield nitration
mixtures which have high water content but involve specific conditions which are difficult to reproduce not knowing the critical details which are now
being better identified.
It is known that in the use of aspirin as a precursor first dissolved in sulfuric acid, a deacetylation and sulfonation occurs leading to salicylic
acid sulfonate as the precursor for nitration. Reportedly this salicylic acid sulfonate should be easy to convert in quantitative yield to picric
acid, via a modified nitration method where the nitration system is a high water content system. This suggests that indeed an aqueous solution of a
mixture of nitrate and nitrite of sodium may be used to accomplish the conversion of the salicylic acid sulfonate in an acidified aqueous nitration
mixture, with the additions of the nitrating agent mixture being possible to accomplished using an aqueous solution of the salts being added or
injected rather than the more tedious method of adding a solid nitrate to a low water content concentrated sulfuric acid solution of the salicylic
acid sulfonate.
 
Attachment: JACS Vol 41 pg2039.pdf (414kB)
This file has been downloaded 643 times
Attachment: US1292266 Picric Acid patent by Datta and Varma.pdf (184kB)
This file has been downloaded 624 times
Attachment: blinded.mid (27kB)
This file has been downloaded 843 times
|
|
|
TGT
Harmless

Posts: 46
Registered: 9-11-2014
Member Is Offline
Mood: No Mood
|
|
This is a little off topic, but recently I have made a batch of picric acid. I have done this numerous times before, but this last time things were
different. When I recrystallize from hot water I usually get fine needles of picric acid after it has cooled. This time for the first time I got
crystals formed okay, but they were not needle like, they just formed at the bottom a flat layer and they became very bulbous and solidified tight to
the bottom of the beaker. Very pretty, but also very different than I am use to. Nothing was changed during the recrystallization. I am wondering
possibly I didn't nitrate hot enough and produced dinotrophenol? Thanks in advance, this has been confusing.
TGT
[Edited on 3-8-2015 by TGT]
[Edited on 3-8-2015 by TGT]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
On the first page of this thread I posted a convenient method for a small scale high yield synthesis of very pure styphnic acid.
A video has been posted which is a slight variation on the method I described tested by another experimenter. I think the process variation shown in
the video would have an improved yield by using the 10 grams resorcinol I specified instead of 8 used for the variation, and the cooling and stirring
reduced with the reaction allowed to run in the initial nitration at a higher temperature as I described.
http://www.sciencemadness.org/talk/viewthread.php?tid=4457&a...
Quote: Originally posted by Rosco Bodine  | Here is a method which I used a few times for styphnic acid which would consistently produce a 90% yield of recrystallized pure product .
10 grams of resorcinol is swirled into and dissolved in 50 ml of concentrated H2SO4 .
The dissolution is moderately exothermic due to the spontaneous sulfonation . After a few minutes a lavender colored precipitate forms and the mixture
is allowed to stand for 2 hours . Then the mixture is cooled to 0 C by ice bath and
nitrated by dropwise addition of 20 ml of HNO3 d 1.4 ~68-70% to the stirred mixture kept below 35C until all is in solution except for small amount of
end product which may be appearing . On recooling to 10C , 20 ml fuming HNO3 d 1.5 ~ 97% is added dropwise to the stirred mixture keeping temperature
below 25C . The stirring is stopped and the mixture allowed to stand in the cold bath for a few minutes . The reaction mixture is then removed from
the cooling bath and the temperature allowed to rise
from the exotherm . Some end product should be seen precipitating at about 28C and the mixture will foam and increase in volume and temperature .
The temperature rise will accellerate at an induction point of about 38C , really accelerating at 45C and from there spiking upward to 75C . Only an
intermittent stirring should be done during the exotherm because the gas bubbles actually dilute the volume density and tend to regulate the reaction
. Stirring the mixture causes the temperature to spike again by reconcentrating the mixture . Only stir periodically when the reaction temperature is
falling and stirring down the foam will kick the reaction and temperature back up again . Generally only stir the mixture if the temperature drops
below 40C from the unaided exotherm of the reaction itself . When the reaction temperature ceases to rise above 35C when stirred , allow the mixture
to stand without stirring for a half hour and then dump the reaction mixture onto 250 grams of ice cubes and rinse the flask with 200 ml ice water ,
everything added together in one crystallizing bowl .
After 30 minutes the mixture is filtered and the crystals rinsed on the filter with about 40 ml ice water . The crude styphnic acid is redissolved in
about 900 ml of boiling water , and on cooling deposits about 20 grams of hexagonal plates of pure very pale yellow styphnic acid . Yield is 90% of
theoretical based on resorcinol . This method works fine for small batches but is possibly not directly scalable upwards without some provisions
for temperature control . For a batch this size , a 500 ml flask is sufficent ,
a 250 ml size is marginal and will threaten to overflow at the peak of the reaction , likely would overflow without any stirring down the mixture
which at times forms a solids filled curd , more than being a liquid consistency . The mixture becomes more liquid again as the reaction completes .
|
https://www.youtube.com/watch?v=Jqfv8qyiKMk&nohtml5=Fals...
<object width=640 height=480><param name="movie"
value="http://www.youtube.com/v/Jqfv8qyiKMk&nohtml5=False?version=3&autoplay=0&showinfo=1&modestbranding=1&controls=1&theme=da
rk&vq=hd720&hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param
name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/Jqfv8qyiKMk&nohtml5=False?version=3&autoplay=0&showinfo=1&modestbranding=1&controls=1&theme=dark
&vq=hd720&hl=en_US&rel=0" type="application/x-shockwave-flash" width=640 height=480 allowscriptaccess="always"
allowfullscreen="true"></embed></object>
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
The styphnic acid produced in the video was far superior to the powerlabs procedure I tried before this one. The synthesis worked very well too and
went pretty much just as you described. There was not a lot of Nox fumes either. The whole process was quite tame unlike The powerlabs procedure
which fumed like all hell and the yield was small.- probably oxidised the shit out of it. I would have used the specified 10g resorcinol but I had
only 8g left. I still have most of the TNR produced leftover and it is nice pale yellow and granular crystals. I expect it to store well and
unchanged for a long time.
. If ever there's a synthesis I want to try I will always look to see if you have posted an optimised version first and your general input about them.
Some of my other videos are based on methods you devised including the mighty Azo-clathrate primary. I think I got it right. I tried to get it as
close as I possibly could at the time. That stuff is quite the primary!
You put alot of thought and effort into your methods and it's always a pleasure to read and try them. Can I ask roscoe what do you do for a living? I
often wonder wher reading your material and think whatever it is you do you must excel at it.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There is in an old Gmelin article reported an interesting double salt of nickel styphnate and potassium styphnate which you should take a look at.
There is a few posts above a description of the usefulness of "nitrous gases" as a nitrating agent, which can be an effect that happens in many
different nitrations where the nitrous gases are supplied from nitrosyl sulfuric acid formed in situ and then controllably very gradually the nitrosyl
sulfuric acid intermediate is decomposed by increasing nitration byproduct water content and temperature of the nitration mixture. On a small scale
nitration it is a method that works very well. The foam collapses and dissipates to a liquid slurry as an indication the reaction is complete.
If the viscosity of the nitration mixture is sufficiently great it produces a stable enough foam, the nitrous gases are trapped and not being lost to
the reaction, the nitration continues in a "blob" of foam that is self regenerating as the reactants are consumed.
That is actually the scheme for the reaction producing styphnic acid so the ratios of reactants are in a narrow range to produce the desired viscosity
that results in that
stable foam as a kind of niche condition for the reaction to follow a specific course.
See the attachment to the linked post
http://www.sciencemadness.org/talk/viewthread.php?tid=11105&...
And another post of interest
http://www.sciencemadness.org/talk/viewthread.php?tid=13283&...
A basic lead styphnate possibly could form interesting clathrates and could also possibly form a neutral double salt with picric acid by boiling 1
molar equivalent of basic lead styphnate with 2 molar equivalents of picric acid. Using 1 molar equivalent of picric acid might result in a
"hemi-basic" lead styphnate - lead picrate double salt. AFAIK such contemplated double salts are purely my conjecture, hypothetical and unreported
compounds, but seem possible. If the "hemi-basic" variant exists, it could also be a clathrate substrate, and might complex with lead azide formed in
situ from an added equivalent of lead nitrate to the reaction mixture followed by slow addition of an equivalent of sodium azide. And if a confirmed
clathrate formation occurs it would possibly complex with more than 1 equivalent of lead azide with a limit of how many more possible, only to be
found by experiment.
With regards to the nickel styphnate - potasium styphnate double salt, when you have basic nickel carbonate or nickel sulfate or other on hand, I have
an idea that nickel hydrazine nitrate due to its extreme low solubility may be easily producible by a different method than usually described using
free hydrazine and nickel nitrate. I think using hydrazine sulfate reacted with calcium nitrate a solution of hydrazine nitrate will be easily
filtered away from the calcium sulfate precipitate, and should react with nickel nitrate in presence of gradually added sodium hydroxide, or sodium
bicarbonate, or ammonium hydroxide, or ammonium bicarbonate used to neutralize the excess nitric acid value associated with the hydrazine nitrate and
form sodium nitrate or ammonium nitrate byproduct remaining in solution. The contemplated reaction might require a separate disassociation of the
hydrazine nitrate done first, freebasing the hydrazine first, using the base separately, before mixing with the nickel nitrate as a better and more
specific sequenced preferential reaction and certain approach likelier to work. The NiHN might be an interesting complementary mixture with the double
nickel - potassium styphnate if that double styphnate salt has the sass as reported by Gmelin.
[Edited on 4/8/2016 by Rosco Bodine]
|
|
|
| Pages:
1
2
3
4 |