| Pages:
1
..
11
12
13
14
15
..
18 |
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I was thinking afterwards that I knew I had seen those same pellets before from an airbag I took apart a few years back. And from what I remember they
didn't make azide, you are correct. As far as the older style azide airbag pellets having other dangerous ingredients, so far I have not seen it. It
has been a while since I looked, but IIRC other than sodium azide and water insolubles there is normally not much else in them except some inorganic
nitrate. I normally have made all my own azide in the past, but sometimes it is just quicker and easier to extract some sodium azide from a few airbag
pellets. In my experience and from what I have read this method is not dangerous, in fact I would say performing an azide synthesis is far more
dangerous to the inexperienced than a simple extraction and metathesis reaction involving sodium azide from airbag pellets. And as primaries go lead
azide, or even silver azide, are very efficient and relatively safe.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by The_Davster  | A few days ago I went to the University library and got an entire 500 page book on inorganic azides. The book gives synthesis of all inorganic
azides(metal and nommetal) and how to crystallize them, electronic structure, lattice dynamics, decomposition, etc..etc. Unfortunalty I do not have a
scanner, otherwise I would upload it to the FTP. So if anyone wants info on any inorganic azide, I'll gladly retype the info that is wanted.
Something that I learned from this book that I did not know before was that there are 3 types of lead azide, with varying degrees of stability.
Keep in mind I only have 2 weeks with this book, but if there is enough demand for info I will renew it. |
Does it contain any methods of synthesizing azides that don't require HN3? Maybe a method that a home chemist could attempt?
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tetra  |
Does it contain any methods of synthesizing azides that don't require HN3? Maybe a method that a home chemist could attempt? |
Yes just search Preparation of sodium azide from n-butyl nitrite and hydrazine hydrate. N-butyl is much prefer than isopropyl , i do both and easiest
is with n-butyl it's just easy to handle. The best reaction is with 80% hydrazine hydrate, but lower % can also do the job.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
What about butyl nitrite makes it easier to handle? I have dkne the reaction wjth isopropyl nitrite but my understanding was that butyl nitrite was
more volitile and harder to handle? Just curious at what advantages you think it has
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
butyl nitrite - bp - 78C
isopropyl nitrite - bp 40C
i done this also with methyl nitrite (is just a gas) generated in situ and it works also good
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Ok thanks, dont know how I thought butyl was the lower boiling. The low boiling is a bit of an inconvenience, I will keep an eye out for any OTC
butanol or heavier alcohols, assuming their nitrite esters are stable and can be used
[Edited on 24-11-2015 by Tdep]
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
working with these nitrites ready for a headache if you do not have a fume hood, I preferred methyl nitrite because it generates directly and
immediately introduced to the reaction time significantly reduced, without the headache, the product comes out very clean, after a few minutes you
will see crystals NaN3 or KN3 coming out from solution, wash with ether, after 5 years i got still very clean, white, dry powder
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
I have got a very basic silly question ! 
If you synthesize lead azid...
Why is everyone using a mechanical stirrer and not a magnetic one.
What are the advantages?
|
|
|
greenlight
National Hazard
   
Posts: 754
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Overhead stirrer prevents formation of large sensitive crystals.
From my view, the magnetic stir bar would cause a cyclone effect in the beaker and suck crystals down into it posing a hazard because of the friction
between the bar and the glass bottom. Lead azide is just as sensitive when wet.
Over head stirrer = no friction between surfaces and doesn't throw the LA against the walls of the beaker like a magnetic stir bar. The newly formed
lead azide falls to the bottom of the beaker away from further risk of initiation.
I also believe you would get smaller safer crystal sizes with the overhead stirrer
Be good, otherwise be good at it 
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
So, this may be a rather amateurish question, but I've read of a method for preparing sodium azide from sodium amide by exposing it to N2O Gas. What
I'm wondering is, would better results be achieved from passing the gas directly over the powder solid, or by bubbling it through a solution?
I found a few things related to this subject, but it seems exceptionally vague or requires already having sodium azide and some kind of very complex
system (https://www.google.com/patents/CA2046385A1?cl=en), or involves making molten sodium amide to bubble the gas into... (https://books.google.com/books?id=YOgDAAAAMBAJ&pg=PA1357...)
Overall, I'm wondering if there's a way to do this without a crazy expensive setup or handling anything more dangerous than the amide itself.
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by James Ikanov  | | So, this may be a rather amateurish question, but I've read of a method for preparing sodium azide from sodium amide by exposing it to N2O Gas. What
I'm wondering is, would better results be achieved from passing the gas directly over the powder solid, or by bubbling it through a solution?
|
Usually passing a gas over a solid is not an efficient way to allow good contact between the two réactants and thus reaction will be
inefficient...using NaNH2 as a solid would mean low temperature (<210°C) and N2O is quite unreactive...so it need T° to allow the reaction to
proceed (see definitions of: activation energy of reaction - energy barrier of reaction - catalyst).
Bubbling through a solution?
I hope you don't wish to use water...NaNH2 is very reactive and dangerous against water and moist air...forming unstable peroxyde explosive crust on
NaNH2.
Maybe you speak of liquid NH3 solution (at -33°C) , then why not... but you stil need the heat to go above the energy barrier of reaction...
Interesting but vague, maybe search harder in this forum with the search tool?
Quote: Originally posted by James Ikanov  |
Overall, I'm wondering if there's a way to do this without a crazy expensive setup or handling anything more dangerous than the amide itself.
|
Reaction of hydrazide (R-CO-NH-NH2) with alkyl nitrite ester (R-O-N=O) in alcoholic NaOH or KOH media is not safe enough for you that you want to try
the industrial process of hot molten NaNH2 with gaseous N2O?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Etanol
Hazard to Others
  
Posts: 190
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
AgN3
Hey, here is a little test of AgN3 and pentaeritritol tetranitrate
C3H5OH + H2SO4 + NaNO2 = C3H5ONO
C3H5ONO + N2H4 +KOH (in C2H5OH) = KN3
KN3 +AgNO3 (in NH3 10%; +CH3COOH) = AgN3



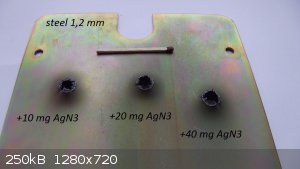
[Edited on 20-12-2015 by Etanol]
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
Quote: Originally posted by PHILOU Zrealone  |
Usually passing a gas over a solid is not an efficient way to allow good contact between the two réactants and thus reaction will be
inefficient...using NaNH2 as a solid would mean low temperature (<210°C) and N2O is quite unreactive...so it need T° to allow the reaction to
proceed (see definitions of: activation energy of reaction - energy barrier of reaction - catalyst). |
Bother, I was hoping that wouldn't be the case.
Quote: Originally posted by PHILOU Zrealone  |
Bubbling through a solution?
I hope you don't wish to use water...NaNH2 is very reactive and dangerous against water and moist air...forming unstable peroxyde explosive crust on
NaNH2. |
Yes, I'm aware, I was hoping to use some other solvent, but none came to mind. I have no access to NH3, so I suppose that's ruled out rather quickly.
Quote: Originally posted by PHILOU Zrealone  |
Reaction of hydrazide (R-CO-NH-NH2) with alkyl nitrite ester (R-O-N=O) in alcoholic NaOH or KOH media is not safe enough for you that you want to try
the industrial process of hot molten NaNH2 with gaseous N2O? |
No, I was hoping to find an alternate method of carrying out such a reaction without the dangers of molten sodium amide simply to see if it was
doable. Making sodium amide "from scratch" reads sigificantly less dangerous than making hydrazine "from scratch". One of my goals was end to end
production of what I use, but it would perhaps be easier for the time being to simply look into what you've suggested.
I'll admit that I used google rather than the search engine, but searching "amide" simply yields every time those 5 letters have been together in that
order.... rather unwieldy.
I appreciate the response!
Edit:
Unrelated, but awesome photos Etanol!
[Edited on 25-12-2015 by James Ikanov]
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
REDOXx
Harmless

Posts: 1
Registered: 23-1-2016
Member Is Offline
Mood: No Mood
|
|
I made a few photos of my last lead azide synthesis. Directly after a successful synthesis:
  
And after 12 hours on the Sun (5mg)
 
The quality is not so good, but I'll try to do better photos. And the synthesis comes naturally also, but I must first translate it from German and
my English is net so good....you know? 
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Found a clean copy of a really good reference on azide technology today, "Energetic Materials Vol. 2 - Technology of the Inorganic Azides". I already
posted this in the short question / quick answer thread, but I thought it was well worth putting here as well. I attached a pdf of "Energetic
Materials Vol. 1 - Physics and Chemistry of the Inorganic Azides", but it is a bit rough and also incomplete.
Attachment: Technology of the Inorganic Azides - Energetic Materials Vol 2.pdf (8.4MB)
This file has been downloaded 3807 times
Attachment: Physics and Chemistry of the Inorganic Azides - Energetic Materials Vol 1.pdf (2.6MB)
This file has been downloaded 1362 times
[Edited on 1-2-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Etanol
Hazard to Others
  
Posts: 190
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Hello, why do not you make a AgN3 directly from the N2H4*H2SO4, AgNO3 and NaNO3 in an aqueous solution?
It works without NH3 at a very slow pipetting of NaNO3 into N2H4*H2SO4 / AgNO3-solution at room temperature and vigorous stirring.
From 3.65g N2H4*H2SO4 and 3.8g AgNO3 in 150...200 ml water and 1.95г NaNO2 in c.a. 20 ml was obtained 1,56 г AgN3.
Time of addition was 4 hours. Perhaps, one can do faster.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Etanol  | Hello, why do not you make a AgN3 directly from the N2H4*H2SO4, AgNO3 and NaNO3 in an aqueous solution?
It works without NH3 at a very slow pipetting of NaNO3 into N2H4*H2SO4 / AgNO3-solution at room temperature and vigorous stirring.
From 3.65g N2H4*H2SO4 and 3.8g AgNO3 in 150...200 ml water and 1.95г NaNO2 in c.a. 20 ml was obtained 1,56 г AgN3.
Time of addition was 4 hours. Perhaps, one can do faster. |
If true, it is a very horrible yield...one would expect something closer to 3 gr...
Maybe has it to do with decomposition of N2H4 in contact with Ag(+) and with unsoluble Ag2SO4 coprecipitation...
In books I have a brief mention of AgNO3 and N2H4 yielding directly AgN3; I have tried but only silver mirror and N2 gas.
I guess there is a typo and that it should be AgNO2 and N2H4...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Etanol
Hazard to Others
  
Posts: 190
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
If true, it is a very horrible yield...one would expect something closer to 3 gr...
Maybe has it to do with decomposition of N2H4 in contact with Ag(+) and with unsoluble Ag2SO4 coprecipitation...
In books I have a brief mention of AgNO3 and N2H4 yielding directly AgN3; I have tried but only silver mirror and N2 gas.
I guess there is a typo and that it should be AgNO2 and N2H4... |
I just recently heard of such a variation of this method. So it was a trial synthesis.I think the yield can be improved.
N2 stands out at too rapid addition of NaNO2 and with stirring is insufficient.
Reaction of silver mirror going with "free" N2H4 (I did't get the mirror ,it was rather the black suspension of Ag )
N2H4-salt gives the desired product.
By the explosive properties the product is identical to that obtained from KN3 and AgNO3.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
How do you separate AgN3 precipitate from Ag and Ag2SO4 ones in the process?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Etanol
Hazard to Others
  
Posts: 190
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
If the process goes correctly, cleaning is not required: Ag is not formed. And AgN3 is less soluble than the Ag2SO4, and former falls in the first
place.
AgN3
water: 0,01g/100g (100°C)
Ag2SO4
0,79 g/100 g H2O (20 °C)
But just in case I dissolved the product in heated water-NH3 (20%), filtered and evaporated NH3.
|
|
|
Great
Harmless

Posts: 34
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Apparently AgN3 sensitivity to static electricity is a total bitch, or at least by the accounts of members in the other thread. I plan on using it
instead of Lead Azide, so can anyone here accurately compare the two? I've got a good impression of shock sensitivities, but less so for static,
heat, light, etc.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This video shows one good general method for sodium azide. IMO the toxicity for NaN3 is understated as a generality and for some individuals the LD
of NaN3 is likely very much smaller. For some materials there is a fairly indefinite range of LD that can vary for the individual.
Also better to use magnetic stirring for the freebasing of the hydrazine from the sulfate to minimize air exposure.
https://www.youtube.com/watch?v=qOSOXVSFdb0
<object width=640 height=360><param name="movie"
value="http://www.youtube.com/v/qOSOXVSFdb0?version=3&autoplay=0&showinfo=1&modestbranding=1&controls=1&theme=dark&vq=hd720&am
p;hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess"
value="always"></param><embed
src="http://www.youtube.com/v/qOSOXVSFdb0?version=3&autoplay=0&showinfo=1&modestbranding=1&controls=1&theme=dark&vq=hd720&
hl=en_US&rel=0" type="application/x-shockwave-flash" width=640 height=360 allowscriptaccess="always"
allowfullscreen="true"></embed></object>
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
cupric azide
In an earlier post (linked) it was described the complex or double salt possible to form from as a mixed salt sort of compounding of 1 molecule
trimethylamine azide with 2 molecules cupric azide to form red crystals exploding 201 C.
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
On you tube has been found a video where someone has been experimenting with small quantities of cupric azide. Although there are many literature
warnings about cupric azide having overt sensitivity and danger, there are reported also investigations that show the material may have usefulness if
the dangers are managed by control of crystallization and special techniques, similarly as would be the case for other reportedly sensitive materials
such as silver fulminate or the like.
Also I think the complexes of cupric azide may have different properties that could provide improvement and reduce the sensitivity of cupric azide so
in spite of the historical bad reputation as would discourage experimentation, with renewed interest in "green" technology materials, it could be that
some modification of cupric azide may be found that is useful in spite of the bad reputation historically that has been earned by the neat cupric
azide alone, or more often reported as attributable to an uncontrolled formation of unregulated crystals as occurs in situ under the special
circumstance of decomposition in storage of lead azide in contact with copper or brass.
The deliberate formation of cupric azide or complexes or modifications by a controlled synthesis may result in a more benign material as the result of
conditions being regulated for the formation of crystals having better physical properties that could make the material have some limited usefulness.
Experimentation with cupric azide and its possible modifications has been limited due to that being discouraged by the historical bad reputation the
material has earned, while full details of all particulars are not found, which leaves it to be a question is such a material in fact worthless due to
the reported danger which has not been full described in all particulars.
It may be that some variant or modification of cupric azide could have value and the specifics have not been determined or described with respect to
what are the required parameters for synthesis and for usefulness as a specific application.
https://www.youtube.com/watch?v=uZz-CQG9qPY
<object width=640 height=360><param name="movie"
value="http://www.youtube.com/v/uZz-CQG9qPY?version=3&autoplay=0&showinfo=1&modestbranding=1&controls=1&theme=dark&vq=hd720&am
p;hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess"
value="always"></param><embed
src="http://www.youtube.com/v/uZz-CQG9qPY?version=3&autoplay=0&showinfo=1&modestbranding=1&controls=1&theme=dark&vq=hd720&
hl=en_US&rel=0" type="application/x-shockwave-flash" width=640 height=360 allowscriptaccess="always"
allowfullscreen="true"></embed></object>
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
It does have a pretty bad rep but I I found the copper II azide to be less sensitive as I first thought.it really is made out to be a monster. That's
not saying it is safe but It was still quite spark and impact sensitive but I'm not so sure about friction. I could not get it to fire when I ground
a small amount between steel surfaces, but I'm sure if I had of added glass or something else gritty and then rubbed it it would have likely fired.
The copper I azide seemed far more sensitive,easily detonating violently even with light taps of the hammer. Some of copper azide complexes do sound
interesting and I would like to try some of these if I can find a decent synthesis for them. I know the tetraamine copper azide can be made by running
a stream of dry ammonia gas over the Cu 2 azide or by dissolving it in ammonia and then evaporation of the liquid with the blue precipitate being the
product. I do know that it does quickly lose the ammonia though and reverts back to copper azide. There's also a lithium/copper azide complex I have
read about that sounds interesting too. Lithium azide presents a challenge though without hydrazoic acid, and I'm not sure the synthesis of this is
something I'm willing to do. It's possible though to make it in a solution of ether or chloroform but again the volatility... A 10% or less
concentration is relatively risk less though
|
|
|
a nitrogen rich explosive
Banned troll
  
Posts: 176
Registered: 28-3-2016
Member Is Offline
Mood: Repentant
|
|
Does anyone have any experience with mercury azides?
Given how far to the bottom right mercury is, I think HgAz would be extremely sensitive and very powerful
I can't think of a better signature.
|
|
|
| Pages:
1
..
11
12
13
14
15
..
18 |