crestind
Harmless

Posts: 23
Registered: 17-1-2013
Member Is Offline
Mood: No Mood
|
|
Formation of Unusual Ammonium Chloride Double Salt?
I found a while ago that sublimating ammonium chloride with iron chloride seemingly causes a double salt of the two to form. In the photo below you
can see how the sublimate has deposited around the top of the beaker (I had a loose lid over the beaker).
NH4Cl + FeCl2 combined sublimation photo
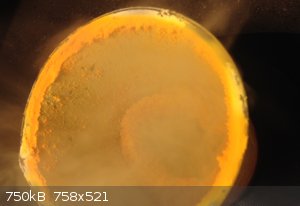
-
When the same process is carried out with common iron oxide instead of iron chloride, the result is somewhat similar in that it takes on a yellowish
orange color. So my question is, in this second instance, is the resulting double salt NH4Cl plus iron oxide, or is it also NH4Cl plus iron chloride
due to the HCl from NH4Cl?
(Unrelated, but if you sublimate NH4Cl with silver chloride, a lot of the silver chloride is carried away by the NH4Cl similarly, but some of the
silver chloride changes to a brilliant red color.)
[Edited on 17-7-2015 by crestind]
[Edited on 17-7-2015 by crestind]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
How'd you find out it forms a double salt?
|
|
|
crestind
Harmless

Posts: 23
Registered: 17-1-2013
Member Is Offline
Mood: No Mood
|
|
Someone told me that was the cause of the orange coloration, the formation of a double salt. It seems to make sense since the resulting product does
contain iron, and neither iron chloride boils at under 1,000f.
It's hard to tell from the photo, but that's a 1,000mL beaker with orange tinted ammonium chloride near the top, so that's almost 6" rise from the
bottom of a beaker.
My guess is that since NH4Cl (decomposition) -> NH3 + HCl, the NH3 somehow forms a temporary iron chloride amine to volatilize it, before reacting
with the HCl gas and condensing again.
[Edited on 18-7-2015 by crestind]
|
|
|
Boffis
International Hazard
    
Posts: 1895
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I am not sure about the product ofthis reaction but the use of ammonium halides particularly the iodide and bromide was a standard procedure for
taking Sn4+ oxide (ie cassiterite) into soultion for analysis. Fused ammonium halides are very corrosive and aggressive and it is very likely that the
sublimate contains a chloroferrate complex but I'll bet there is a large excess of ammonium chloride.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | | I am not sure about the product ofthis reaction but the use of ammonium halides particularly the iodide and bromide was a standard procedure for
taking Sn4+ oxide (ie cassiterite) into soultion for analysis. Fused ammonium halides are very corrosive and aggressive and it is very likely that the
sublimate contains a chloroferrate complex but I'll bet there is a large excess of ammonium chloride. |
That would be my guess too. But the colour does point to Fe(+3) (yellow ferrous compounds are rare) and oxidation of Fe(+2) to Fe(+3) in these
conditions is likely.
|
|
|