| Pages:
1
..
17
18
19
20
21
..
33 |
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Yeah, sorry...you were talking about crystallizing ammonium DNAc directly from the neutralized nitration mix, (compact red crystals) while I was
talking about recrystallizing ammonium isopicramate (bulky deel purple crystalline mass). Since the high temperature deacetylation under partially
aerobic conditions also is likely to produce some impurities, it seemed more logical to start there. After soms more consideration, even the
diazotization producers impurities, so in order to get highly pure ddnp (both for o and p), i reckoned crystallizing the ddnp from acetone would be
most efficient.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Here is an interesting read on paracetamol. Synthesis and purification is discussed.
Attachment: Paracetamol - A Curriculum Resource.pdf (1.4MB)
This file has been downloaded 1993 times
[Edited on 20-6-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by nitro-genes  | | Yeah, sorry...you were talking about crystallizing ammonium DNAc directly from the neutralized nitration mix, (compact red crystals)
|
The neutralization of the quenched nitration mixture from the ammonium nitrate plus sulfuric acid nitration method might possibly not go the way I
hope, however I still think it likely will occur as predicted. The proof will be in the pudding. It will depend upon whether or not the ammonium
bisulfate (and sodium bisulfate) is a stronger acid than the acetylisopicramic acid, which I am honestly really just guessing is the case. But I
think it is a good guess 
Edit: See several posts below where a further review of this neutralization shows that using a sodium value alone will not work to free resident
ammonia to form the ammonium acetylisopicramate in situ, but a neutralization using an ammonia value will be required, as in the "Plan B" also
considered as more certain produce the ammonium acetylisopicramate.
A controlled neutralization of the quenched nitration mixture by gradual addition of a sodium value in solution as hydroxide or carbonate or
bicarbonate or sesquicarbonate (whatever is convenient) should result in a sequential neutralization of the acidic components present in the nitration
quenched mixture in the following order of neutralization [1] H2SO4 [2] HNO3 [3] HN4HSO4 [4] acetylisopicramic acid [5] any unreacted NH4NO3
The neutralization of [3] to form (either) sodium bisulfate or the normal sodium sulfate will produce byproduct ammonia which should be sequestered by
neutralization of [4] forming ammonium acetylisopicramate.
Edit upon review: What will actually occur here is the ammonia evolved from an ammonium acid sulfate being neutralized by a sodium value will simply
attach to any adjacent ammonium acid salt to form the neutral ammonium salt.
System component [4] would tend to be the absorber for any byproduct ammonia up to the point where all the acetylisopicramic acid has been neutralized
by forming the ammonium salt. At that point of neutralization no more byproduct ammonia can be absorbed and the free ammonia evolving should be
easily detectable by its odor. The detection of ammonia would there mark the completion of neutralization, and when the solution cools the ammonium
acetylisopicramate should crystallize out as the only precipitate.
If the neutralization scheme I have contemplated is not correct based upon my guess about the bisulfate, then Plan B would involve a rough just under
theoretical neutralization of the estimated free mineral acid values using a sodium value, followed by doing the remainder of the neutralization with
an ammonia value like ammonium carbonate, bicarbonate, or hydroxide. From there things should proceed as contemplated otherwise, 100% guaranteed.
Plan A will likely work okay and if not, then Plan B for 100% positive certain should work.
Edit: Please note that a further review shows that the "Plan A" first contemplated will not work, but Plan B should work fine.
Both these schemes are the result of effort to achieve economy on use of an ammonia value for the complete neutralization of the quenched nitration
mixture. The best that can be done is to use a sodium value for neutralization of the system components where an ammonium salt is not needed or is
already present, and reserve the use of more expensive ammonia for the neutralization of the acetylisopicramic acid to faciliate it isolation by
crystallization.
| Quote: |
while I was talking about recrystallizing ammonium isopicramate (bulky deel purple crystalline mass). Since the high temperature deacetylation under
partially aerobic conditions also is likely to produce some impurities, it seemed more logical to start there. After soms more consideration, even the
diazotization producers impurities, so in order to get highly pure ddnp (both for o and p), i reckoned crystallizing the ddnp from acetone would be
most efficient.
|
I can predict some of what I think should occur according to educated guess and theory. But experimental result is the essential verification. IMO a
direct deacetylation of the ammonium acetylisopicramate could work fine and eliminate an intermediate isolation step that may not be needed.
The subsequent dilution of the deacetylation mixture will contain ammonium bisulfate as a spectator ion. The neutralization by a sodium value should
follow the neutralization sequence [1] H2SO4 [2] NH4HSO4 [3] isopicramic acid
At the point where the incoming sodium value has neutralized [1], it is expected that likely all of the isopicramic acid will precipitate as the free
acid. As incoming sodium value continues and some [2] is neutralized, the byproduct ammonia reacting with trace isopicramic acid in the supernatant
liquid should cause a purple marker endpoint showing the appearance of the ammonium isopicramate. Adding back a little acid as H2SO4 or HCl should
make the purple color disappear again.
This should mark the endpoint for the neutralization of the deacetylation solution. The precipitated isopicramic acid is filtered out and dried.
P.S. That's some of the reaction map description you were asking me about before.
I need the formation and dilution data for the isopicramic acid sulfate to be able to calculate the dilution volume and acidity that is needed for
keeping everything in solution in the diluted deacetylation mixture, which should result in a clean separation and precipitation of the pure
isopicramic acid.
At this point it should be noted that I believe it is very likely a technical grade of p-DDNP could be obtained from diazotization of the diluted
deacetylation mixture, either prior to any neutralization, or after a partial neutralization that may leave in solution the still soluble isopicramic
acid. In a bulk process such as an industrial scale where the p-DDNP may be destined to be used as an explosive fuel component and sensitizer for
ammonium nitrate or ammonium perchlorate as examples, perhaps with the p-DDNP dissolved in nitromethane or other solvent, such a technical grade of
p-DDNP may be adequate,
and the step of isolation of the isopicramic acid would simply be skipped for the production of technical grade p-DDNP by diazotization of the
deacetylation mixture.
If further purification is needed the isopicramic acid can be dissolved in HCl as soluble isopicramic acid hydrochloride and then diluted and
neutralized with a sodium value to precipitate again the low solubility free isopicramic acid.
I think that understanding these particulars and applying them as I contemplate is possible, manipulations can be done using fairly heavily loaded
solutions comfortably less than saturated, and still get clean separations with minimal transfer losses.
There is an idea I have for a diazotization method that could be interesting. Dabney reports that the potassium salt of isopicramic acid is extremely
soluble in water, and possibly the sodium picramate is likewise extremely soluble in water and could work as well. My idea is to mix cold aqueous
solutions of sodium or potassium isopicramate with a cold aqueous solution of sodium nitrite, and to the stirred mixture very slowly add an acid such
as acetic acid, hydrochloric acid, ect. which may cause the diazotization to proceed very gradually and allow for a crystal growth of the p-DDNP even
in a cold reaction system, if there is a reaction between the isopicramate value and the nitrite value that proceeds concurrently as the acid is
added, rather than a sequential reactivity. There is a chance this diazotization scheme could work or it may not work. Only an experiment can
determine if this idea for the diazotization has validity.
Ha! The "stealth wizard" strikes again!
http://www.sciencemadness.org/talk/viewthread.php?tid=14342&...
Ditto!

I miss the posts of a few old friends who I think would probably have been posting in this thread as in many times past, if they were still among the
living in this world. So this thread has been a kind of sad "roll call" for me where many old friends probably gone from this world have not
answered.
[Edited on 6/21/2015 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  |
There is an idea I have for a diazotization method that could be interesting. Dabney reports that the potassium salt of isopicramic acid is extremely
soluble in water, and possibly the sodium picramate is likewise extremely soluble in water and could work as well. My idea is to mix cold aqueous
solutions of sodium or potassium isopicramate with a cold aqueous solution of sodium nitrite, and to the stirred mixture very slowly add an acid such
as acetic acid, hydrochloric acid, ect. which may cause the diazotization to proceed very gradually and allow for a crystal growth of the p-DDNP even
in a cold reaction system, if there is a reaction between the isopicramate value and the nitrite value that proceeds concurrently as the acid is
added, rather than a sequential reactivity. There is a chance this diazotization scheme could work or it may not work. Only an experiment can
determine if this idea for the diazotization has validity.
I miss the posts of a few old friends who I think would probably have been posting in this thread as in many times past, if they were still among the
living in this world. So this thread has been a kind of sad "roll call" for me where many old friends probably gone from this world have not
answered.
|
From what I have seen in the diazotization of o-picramic acid (as in the method from COPAE) or the diazotization of the sodium salt (as in the method
from DDNP a Detonating Explosive) the picramic acid or sodium picramate is a slurry with only partial dissolution and when the final reactant is added
(usually sodium nitrite solution) the solid picramic acid or sodium salt is dissolved and consumed as the reaction progresses and replaced with a
slurry of DDNP. The method starting from sodium picramate is a much slower reaction, which does make a more free flowing product.
Yeah, even in the seven years or so I have been reading these threads a lot of good old guys have vanished. Not completely gone though, a lot from
them was transferred to others who continue to use it and carry it forward.
[Edited on 20-6-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yes I understand the scheme used and described for variations before, but this is simply an idea that could work which has not been described before
to my knowledge. Using the very soluble salts together in a more highly diluted system that would have two soluble reactants very incrementally
brought into reaction to form a nearly insoluble product very slowly and gradually could benefit crystal growth in the cold. It just seemed that the
incoming acid might have no particular preference for displacement of sodium from one component or the other, and would then result in a smooth
diazotization favoring crystal growth. As opposed to a slurry I would think a very dilute reaction system of dissolved precursors would be preferred,
where the only thing most likely to be precipitating is the growing crystals of end product uncontaminated by any about as insoluble undiazotized
precursor like isopicramic acid. The isopicramic acid has much lower solubility than picramic acid, so i was thinking the modified method might work
better, but it is an unknown how it may actually perform.
Yeah its sad all the missing members,
like Axt, and quicksilver, and ordenblitz,
Blaster, Boomer, and Engager and The WiZard is In
All of them missing for awhile and even Len too ....
(also known affectionately) as
I nicked him "Doctor Meteor Shower" 
I feel something like the last Mohican.
[Edited on 6/20/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Thanks for your ideas about performing the reactions. The more I think about it, using AN for the nitration and then adding a strong carbonate
solution for precipitating the ammonium salt of DNAc could be a very good idea for retrieving a larger and potentially more pure yield of ammonium
DNAc for subsequent deacetylation!  NaOH might also work, but since one patent
mentioned that the temperature during neutralization should remain below 30 deg C, it will take a long time to make the total addition. A lot of heat
will probably dissipate with the CO2 produced, making this the best option IMO. I'm quite sure that A-bisulfate is a much stronger acid than
isopicramic, will the carbonate dissplace enough ammonia to provide an olfactory sign for completed neutralization? NaOH might also work, but since one patent
mentioned that the temperature during neutralization should remain below 30 deg C, it will take a long time to make the total addition. A lot of heat
will probably dissipate with the CO2 produced, making this the best option IMO. I'm quite sure that A-bisulfate is a much stronger acid than
isopicramic, will the carbonate dissplace enough ammonia to provide an olfactory sign for completed neutralization?
With acidicity increasing during diazotization from K-isopicramate and KNO2, the free isopicramic will precipitate from the solution pretty quick when
the pH is lowered by adding the acid, so the outcome is more complicated than that. I already tried this synthesis, but than at higher temperatures
(30 deg C), according to the chinese article, but this gave a very impure product and dark brown filtrate. Attempting this at 0 deg C would be a
better option.
Also tried to determine the concentration of SA that still dissolves all the isopicramic. At 20% SA, almost no dissolution takes place even with
supplemetal heating. I used 3 grams of isopicramic, and added 20 ml of a 50/50 v/v 98% SA/dH2O mix. After some heating everything seemed to dissolve,
but this also turned out sort of a minimal concentration to keep everything in solution at RT. Upon dilution with distilled water, from there adding 2
ml of water, slight clouding could be seen and, further dilution will pretty much precipitate out most of it. It is VERY difficult to see though,
since the solution is VERY dark in colour, so I used a strong light to be able to see something. Maybe, it is best to add no water at all after
deacetylation and directly start neutralizing with ammonia right away. I'm very sure I didn't see precipitation to this extend the first time though,
since I added an equal volume of water then and upon filtering I didn't see more than maybe 10-50 mg of insolubles.... confusing.
The document Henning posted is also interesting, there they perform the deacetylation of acetaminophen in 1M SA and heat at reflux for 1 hour. The
DNAc should deacetylate more easily, so this should work and can be performed in an airtight vessel, perhaps leading to less oxidation (if there is
any) during deacetylation. Disadvantage would be that the solution can't be filtered, but would mabe be less of a problem when pure Ammonium DNAc is
started out with. 
Also performed the diazotization of 2 grams of isopicramic acid in 40 ml 10% acetic acid (stronger would probably be better since solubility of the
DDNP will increase, but was not available), and 0.75 grams of NaNO2 at 0 deg C. It is incredibly slow compared to using strong acids, taking a good 30
minutes before the colour shift so yellow/brown became apparent. It is drying right now, but very curious how the p-DDNP will turn out when diazotized
this way.
[Edited on 20-6-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There is definitely enough ammonia value present for the Neutralization scheme "Plan A" to work if indeed Plan A does work as contemplated. It is the
"if" that is the issue there. 
As it turns out on further review I discovered I overlooked something simple.
I have been looking at this more and I see a problem there with my first contemplated neutralization scheme "Plan A", because there will be produced a
sodium acetylisopicramate
instead of the hoped for ammonium salt, if there is only a sodium value used for the neutralization of the quenched nitration mixture. What would
occur is a mixture of neutral ammonium and neutral sodium salts would form preferentially to evolution of ammonia from any decomposition of the
ammonium salts. Any ammonia evolved from an ammonium acid salt decomposed by a sodium would simply attach to another ammonium acid salt and form the
neutral ammonium salt, and would not evolve to be absorbed by the acetylisopicramic acid. I just didn't see this reaction on first look at the
reaction system. I can sure see it now and it is simple so I really should have seen it before. I have other tasks presently as a distraction so
please pardon my oversight on this point involving the neutralization of the quenched nitration mixture. Plan B the alternative should work though.
Reviewing what I had described before as Neutralization "Plan B" is positively the more logical approach to the certain formation of the ammonium
acetylisopicramate. Doing the initial "undershoot" near neutralization of the theoretical quantity of free mineral acid values in the spent nitration
mixture, plus an equivalent sufficient for conversion of just half the acid sulfate salts to neutral salts using a sodium value, (the
already resident ammonia will form neutral salts of the remaining half the acid sulfate salts present) and then following that using an ammonium value
in excess. That is the 100% sure to work method there. So is also certain to work doing the entire neutralization using ammonia, but more expensive.
I still haven't completed the reaction equations and really Plan B is the more conventional approach there.
I'll look at this again because it isn't yet settled in my own mind.
After reviewing this I have made Edit notes and corrections to my earlier post above while allowed time to edit.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
Review of this neutralization of the quenched nitration mixture shows that a sodium value can be used for only the bulk portion of neutralization,
which will need to be completed using an ammonia value to produce the ammonium acetylisopicramate. For the ammonium acetylisopicramate to form in
situ from byproduct ammonia from displacement by sodium salts is not likely.
On the other matter regarding the isopicramic acid sulfate, based upon the solubility test done by nitro-genes,
formation of the soluble "acid salt" of isopicramic acid requires an extreme low pH condition for H2SO4 that is more than a 50% strength by weight of
H2SO4.
You should believe what your own experiment has shown you and apply that information to calculating what is an ample dilution volume and pH to be
provided for the deacetylation step.
You reported figures showing you used 5 ml of 50/50 v / v H2O/H2SO4 per gram of paracetamol then dinitrated and deacetylated, and you may see
improvement increasing that to 6 or 7 (or more) that may be fine, based on experiments to follow and determine effect of such adjustments. If the
deacetylation is done upon the ammonium acetylisopicramate as I have described should work, an additional equivalent fractional molar amount of H2SO4
will be required to neutralize the ammonia as ammonium sulfate with the remaining usual amount of H2SO4 functioning in the actual deacetylation of the
freed acetylisopicramic acid become available from its ammonium salt.
The same pH related solubility scenario with HCl as a comparison would be good to know as well, for the purification step decribed by Dabney using HCl
to form the soluble acid hydrochloride salt.
Based upon that information about the H2SO4 soluble acid salt then it would be best to use an ample plenty of 50/50 v/v H2O / H2SO4 for the
deacetylation heating to assure that everything dissolves when hot. And for a dilution in advance of neutralization I would recommend then pouring
the still hot deacetylation solution in a small stream into a perhaps 6 to 10 times additional volume of stirred and nearly boiling hot water. Upon
cooling a lot of free crude isopicramic acid should be precipitated by the dilution alone, and this should be filtered out and set aside as the main
harvest and perhaps the complete yield.
Neutralization of the cool diluted deacetylation mixture is likely not going to recover much of anything additional but it could be gradually
neutralized as an experiment just to confirm no further quantity of isopicramic acid is being held in solution as acid sulfate.
Dabney reported that the purification of isopicramic acid is best done by dissolving in HCl in which the impurities do not dissolve.
Reasonably, the manipulation thereafter would be filtering the HCl solution from impurities, and recovering the isopicramic acid by dilution and
neutralization of the HCl solution to cause precipitation of the low soluble form of free isopicramic acid.
Dabney reported the isopicramic acid is very easily soluble in alcohol. So not only is alcohol a candidate purification recrystallization solvent, it
may also be possible to diazotize in alcohol by introducing "nitrous gases" produced from HNO3 plus starch as a gas generator, or in the alternative
to diazotize using an organic nitrite added to the cold alcohol solution of isopicramic acid.
I was mistaken earlier about the first neutralization scheme
contemplated for the quenched nitration mixture, leading to a desired ammonium salt of isopicramic acid. The neutralization can be done only part
way using a sodium value to do the bulk part of the neutralization for economy of reagents, but there is required an ammonia value for neutralizing
the acetylisopicramic acid to obtain the ammonium salt.
[Edited on 6/21/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
I was thinking, maybe it doesn't need complete neutralization at all.  Maybe
adding ice and a very strong solution of sodium sulfate (maximum about 1 mole per mole of sulfuric used can lower the solubility of the DNAc or
potential acid soluble compounds, without changing pH leading to an exotherm. Sort of salting out procedure. Can't find a solubility chart for sodium
bisulfate, but according to Sigma-Aldrich it is 390g/L at 16 Deg C, meaning it would likely stay in solution. Maybe
adding ice and a very strong solution of sodium sulfate (maximum about 1 mole per mole of sulfuric used can lower the solubility of the DNAc or
potential acid soluble compounds, without changing pH leading to an exotherm. Sort of salting out procedure. Can't find a solubility chart for sodium
bisulfate, but according to Sigma-Aldrich it is 390g/L at 16 Deg C, meaning it would likely stay in solution.
The attempted 10% acetic acid diazotization produced an amorphous fluffy DDNP with more carbon deposits, no benefit there it seems, maybe stronger AA
would be better.
The isopicramic when recrystallized from methylated spirits is really one of the most beautiful compounds I've seen yet. It is almost like pure gold
foil. (The imprints are from blotting dry with paper towels). Picture of 3 gram (test run) recrystallized ispicramic attached. The earlier mentioned
12 grams solubility per liter is probably inaccurate, it may be that the insolubles are actually impurities that remain behind and crystallize earlier
from solution in the cold. When filtered off and boiled to nearly dryness, this remained, which corrseponds to Dabneys report on the gold lustre. So
far it keeps the gold lustre very well, no sign of surface oxidation yet. It is not known what is in the methylated spirits, I just hope that it isn't
some compound formed from heating of the solution with isopicramic. Ah well, OTC is gold...

Almost seems like a waste to diazotize 
[Edited on 22-6-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Very nice, the purified isopicramic acid has a shiny golden luster like a gold bronzing pigment. You should save a vial of the material as a pH
indicator good for mineral acid titrations and perhaps good for other special things.
On the neutralization, I agree. When you have 90% or more of the product crystallizing out all by itself, then it makes sense to just filter out the
easily gotten material which is the main yield and then do any neutralization scheme to try to recover any remaining yield that may have been in
solution. The Casseellas patent GB24409 described just filtering out the acetyl derivative directly from the quenched and diluted nitration mixture.
And with that nitration scheme there was no help for the precipitation from any salting out by a byproduct ammonium bisulfate.
[Edited on 6/22/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Thanks, also would be interesting to see what the product will be after diazotization. Hopefully, this will really give an indication how much
impurities are formed during diazotization of p-DDNP and nitratio/deacetylation.
The nitration can probably still be optimized, as well as the deacteylation, IIRC GB24409 mentions 90% yields after nitration indeed. It is a lot of
hassle to isolate the DNAc as the stable free and drie acid without remaiing SA.Only tried once, by trying to wash with a very dilute ammonia
solution in order to estimate yield, and it seemed there is room for improvents. On the other hand, maybe the extended washing took a great deal of
product back into solution.
If I do the complete synthesis again, I have some things that I will alter:
1. After nitration, adding crushed ice and after melting add a 30 deg C solution of 40% Na2SO4 to try and salt the remaining DNAc out.
2. For the deacetylation: either 3 days at room temperature in 50 ml 50% SA (v/v), or just performsing the reaction in an airtight container
3. Adding hot water slowly to the deacetylation mix instead of ammonia (would be really cool of that works as good or even better as ammonia)
You mentioned that you were going to try the nitration according to the patent above yourself, any results yet? 
[Edited on 22-6-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
You were asking about the solubility for ammonium bisulfate and it is high to the extent a 50% solution can exist in the cold. There is possibly a
specific concentration of H2SO4 in which the acetylisopicramic acid is least soluble, and that would be the dilution to seek if it that holds true and
once the concentration is identified. The principle is exploited for picric acid when the nitration mixture is diluted to a specific volume at which
concentration the solubility is so low there is almost no loss because all of the picric acid is precipitated. I don't recall exactly what it was
maybe 20% H2SO4, I'll see if I can dig it up because it might hold true for other materials like this.
I don't favor adding the neutral sulfate because of the glauber salt to be advoided if possible. The neutral sulfate readily forms a decahydrate
which is voluminous and solid and can be a problem.
I wish I could do the experiment but it will have to wait for freed up time when I can get to the experiment which is the fun part. I have a couple of
months worth of other business that won't wait because of a deadline in August. Another backlog of other chores waits now, including assembling three
upgrade computers.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
One curious thing I want to look into is whether an insoluble carbonate salt/adduct can be formed from CO2 and dinitroacetaminophenol. Sounds strange
maybe, but upon neutralizing part of the nitration mix with sodium carbonate, a bright yellow intermediate compound seemed to be formed. When adding
ammonia to this compound it seems to release CO2 again and take on an orange colour again. This happened very early in the neutralization, under very
acidic conditions. Very curious, any thoughts on this? This is based on one observation only, but if true maybe this could be exploited to isolate the
DNAc in more pure form.
[Edited on 23-6-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It can be some involved reaction algebra to sort out what is occurring maybe a double salt with one ammonium salt and one sodium salt. I don't know.
And the reaction where I was thinking the lowest solubility ammonium salt might ultimately be precipitated as a result of ammonia neutralizing the
acetylisopicramic acid I later saw would not happen that way because a sodium acetylisopicramate would be the first to form. However, if the
solubility of the sodium salt is great while the solubility of the ammonium salt is low for awhile some of the dissolved sodium salt might be
decomposed by the ammonia and some ammonium salt precipitate. Generally a sodium will displace an ammonium, but a solubility driven reversal could
occur, and it can depend also depends upon the amount in excess the concentration of the sodium or the ammonia, which can drive the reaction to
preferentially form one or the other. These systems are nuanced and difficult to predict as abstracts, and really the only proof is to see what
occurs. It can also be temperature driven and at one temperature the equilibrium for a reaction goes one way and at another temperature just the
opposite. Likewise for pH. Some general predicitions can be made but there can be surprises.
When I was first looking at the neutralization of the quenched nitration mixture from a NH4NO3 + H2SO4 I was thinking that even if the sodium
acetylisopicramate did form, it is much more soluble than the ammonium salt.
Simplifying, for example you mix two solutions of highly soluble compounds, one solution is ammonium carbonate or bicarbonate and the other is sodium
acetylisopicramate.
Ammonium Acetylisopicramate should precipitate from the byproduct solution of sodium carbonate or bicarbonate.
Well, suppose the sodium acetylisopicramate does form as the first stage neutralization of the quench nitration mixture to the point of neutral
ammonum sulfate and sodium sulfate, but incoming base addition is continued so that the sodium value decomposes the neutral ammonium sulfate, and
produces in situ the ammonium carbonate or bicarbonate byproduct which subsequently indeed does react with the soluble sodium acetylisopicramate to
precipitate ammonium acetylisopicramate.
What I was first thinking as the ultimate product of neutralization by a sodium value could theoretically occur, but will it?
Only an experiment will show whether it does occur that the ammonium acetylisopicramate is indeed formed from the indigenous ammonia. It could still
work, but simply require additional base needed to decompose the neutral ammonium salts.
However a potential issue that could arise is the possible decomposition of the acetylisopicramic acid if it is sensitive to decomposition by an
excess of base. In that case it is possible that the stable sodium acetylisopicramate could be decomposed by the scheme trying to convert it to the
ammonium salt. It is unknown if the contemplated reaction scheme would work wonderfully to actually produce the ammonium salt or if the end result
would be decomposed "red goo". Experiments would be required to see what actually does occur. It is theoretically possible it could work very well or
not work at all.
It would be surprising to me if the acetylisopicramic acid has the hydrolytic stabilty to withstand being subject to a warm basic system in solution
that would accomplish that scheme for its precipitation as a subsequent reaction. My concern is that the reactions which work in theory may not be
reducible to practice because of the sensitvity to high pH of the acetylisopicramic acid. But what actually may happen there is simply unknown. It
would be the hydrolytic stability of the acetylisopicramic acid which is governing whether the contemplated neutralization scheme would be possible or
not.
Another possibility for the neutralization is that the scheme works but the reaction doesn't have a favorable equilibrium to run to completion and a
mixed product results like a double salt or coprecipitate, or the process works but has attendant partial decomposition in which case an impure
product can result. So the "process engineering' becomes complicated and really the only sure way to tell what will occur is to actually run the
theoretical synthesis and see what the run produces.
The temperature can have great bearing and so can the dilution and the pH, so the same general synthesis can produce different results for different
reaction conditions.
There are many patents which are related to an identification of specific reaction conditions which produce a desired result from a reaction system
which does not occur for reaction conditions that are not within a narrow range. This illustrates the principle of a "patent process" which someone
has discovered by sometimes many experiments.
Regarding the neutralization scheme as a modified scheme of synthesis to economize the neutralization process, but still having a high probability of
success and least uncertainty, I think the "Plan B" neutralization scheme using a reduced amount of ammonia by first using a sodium value to
accomplish the bulk of the neutralization, followed by use of an ammonium value to cause a first neutralization of the acetylisopicramic acid is the
best approach. The ammonium acetylisopicramate would be then deacetylated directly using a bit more H2SO4 for reason of the ammonium component.
An alternative is to use no neutralization scheme for the quenched nitration mixture, but first to filter out the free acetylisopicramic acid that
does precipitate on first dilution, and set this main yield aside, and then perform any neutralization on the remaining spent nitration mixture
mixture to try to recover any acetylisopicramic acid held in solution, to be isolated as the ammonium acetylisopicramate. This would reduce the
ammonia requirement further since the bulk of the producrt will be left as free acetylisopicramic acid and only the recovered amount remaining
dissolved in the spent nitration mixture will need ammonia for its neutralization.
The bulk material filtered which was gotten first from dilution alone of the nitration mixture, can be added together with any ammonium
acetylisopicramate gotten subsequently from a neutralization, and the mixture can be deacetylated.
The economics favor this combination scheme where in part the yield is obtained from no neutralization but only dilution of the spent nitration
mixture, and subseuently any neutralization is done as a recovery scheme for whatever product did not precipitate on dilution. If no reasonable
amount of material is recovered by the neutralization then there is no benefit from even doing a neutralization on the spent nitration mixture.
[Edited on 6/23/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
1 gram of the purified isopicramic acid (IPA) was diazotized using three different methods, stirring was continued for 30 minutes after the final
addition for all 3 methods:
1. Suspending 1 gram of IPA in 12 ml 6% HCl at 0 deg C. Adding a solution of 0.36 grams of sodium nitrite in 4 ml dH20. (COPAE) This produced an
instant colour change towards yellow/browish. Diazotization of isopicramic is MUCH faster than for picramic acid. Dried product is yellow/brownish,
matt looking fine powder that burns with moderate carbon deposits. Adding a small amount to acetone indicates unreacted isopicramic impurities.
2. Dissolving 1 gram of IPA in 20 ml dH2O + 0.2 gram NaOH at 0 deg C --> stirring for 10 minutes producing a dark red/violet solution/suspension of
sodium isopicramate. Thereafter, 0.36 grams of sodium nitrite in 4 ml water was added and 15 ml of HCl was slowly allowed to drip in over the course
of 45 minutes. Product is more dense and has a silver sheen, but is a dark brown in colour and produces much more carbon deposits than the other
methods. DIssolving a spatula in acetone produces dark brown solution
3. Same as method 2, only the HCl was added at once instead of dripping in. Immediate colour change, more dense product than method 1, more orange in
colour and slight glistening indicating larger crystals. This seems to produce the best pDDNP of the three methods, producing the least amount of
carbon deposits.
It seems that optimal diazotization of isopicramic needs other conditions than o-DDNP
[Edited on 25-6-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
One of the diazotization in alcohol schemes could be needed.
There is a description of various methods found here starting at page 6 of the book. Page 11 and 12 regarding the quinone diazides is most pertinent
https://books.google.com/books?id=XYhY9AUzVD0C&pg=PA4&am...
4-6-dinitro-quinone-2-diazide is synonymous with o-DDNP
2-6-dinitro-quinone-4-diazide is synonymous with p-DDNP
Here is a screen grab excerpt from the book linked above

In a previous post I mentioned the compound [8] of the Klapotke article is an isomer of one of the compounds identified by the Von Herz patent
GB207563 for which the potassium salt is reported by Von Herz to have initiating power at least equal to lead azide and detonates in the smallest
quantity. I am still looking at this because Von Herz says there are two isomers which are substantially the same in performance. It may be that the
compound [8] of the Klapotke article is identical to the patent compound of Von Herz. In either case if it is identical or the isomer, the potassium
salt should be extremely useful as reported by Von Herz in GB207563.
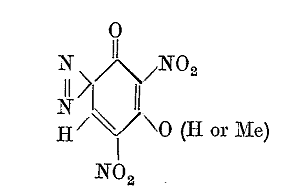
We have an easier route to the compound [8] of the Klapotke article and / or the Von Herz patent by use of paracetamol as a starting material first
converted to the mononitrate, the 3-nitro derivative, which is separated, and then further nitrated to obtain the 2,3,6 trinitro derivative.
I believe that the 2,3,6 trinitro compound will result if the same nitration is performed as is used to make acetylisopicramic acid, but instead of
using paracetamol for the nitration mixture, the 3-nitro derivative of paracetamol is subjected to the same nitration mixture.
In the alternative the same slow nitration method Klapotke applied to compound [5] which is paracetamol acetate may likewise serve to convert
3-nitroparacaetamol to the 2,3,6 trinitro derivative.
A deacetylation procedure was used by Klapotke which was done very slowly at ambient temperature, first dissolving the trinitroparacetamol in sulfuric
acid at 0C, and then at ambient temperature stirring in an open flask for 3 days, presumably evaporating the by product acetic acid, and then pouring
the mixture onto ice to precipitate the product.
Diazotization was accomplished simply by dissolving the trinitroaminophenol in ordinary concentrated HNO3 and warming to 65 -70 degrees with stirring
for 2 hours and pouring the mixture onto ice to precipitate the compound [8]
Attachment: GB207563 Diazodinitroresorcinol.pdf (371kB)
This file has been downloaded 479 times
Attachment: A NEW NITRATION PRODUCT, 3-NITRO-4-ACETAMIDOPHENOL, OBTAINED FROM ACETAMINOPHEN WITH NITROUS ACID.pdf (178kB)
This file has been downloaded 535 times
Attachment: Synthesis & Energetic Properties of 4-Diazo-2,6-dinitrophenol and 6-Diazo-3-hydroxy-2,4-dinitrophenol.pdf (286kB)
This file has been downloaded 1074 times
So there you go, if Edmund von Herz knew what he was talking about in May of 1922, and the Japanese pharmacists knew what they were talking about in
1989, then it should be easy enough to make from ordinary paracetamol without much difficulty or expense, a green energetic initiator that is equal in
power to lead azide, and that would be
potassium 4-diazo-2-6-dinitro-resorcinol-(1-anhydride)
This is the diazo derivative of styphnamic acid which can be made by a different route
Attachment: from JCS 1881 Styphnamic Acid related pg1133.pdf (303kB)
This file has been downloaded 519 times

This same compound had caught my attention before and was mentioned in an earlier post
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
The path to the same compound via styphnamic acid via diazotization analogous to picramic acid is described in US4246052
Attachment: US4246052 SnCl2 reduction of Styphnic Acid and DDNP analogue therefrom.pdf (240kB)
This file has been downloaded 529 times
The same diazodinitroresorcinol can be made more easily and economically from paracetamol
[Edited on 6/26/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Great finds Rosco! Initiating power equal to lead azide for the potassium salt of DDNR sounds great, looks like it can be recrystallized from water as
well. Difficult part will undoublly obtaining the dinitro aminorescorcinol, and SnCl2 to perform the reduction.
The fun with these compounds just doesn't seem to stop. Apart from the fact that paracetamol makes an easy route to isopicramic acid and a word class
primary(ies?), the isopicramic acid also seems to crystallize as an almost pure gold compound...and burning it is very funky. Burning one gram
reminded me somewhat of mercury(II)thiocyanate, producing these organic looking "branches" of carbon when it is burning. Picture attached, it was even
better looking before I tried to move it and part of it fell apart. 
Oh, also did an experiment with diazotization in ethanol by the following procedure:
1 gram of recrystallized isopicramic acid was dissolved in 50 ml of ethanol (gentle heat applied to speed things up). This was placed in an ice bath
and allowed to cool to about 5 deg C again, after which 1 ml of 98% sulfuric acid was added (no sign of precipitation) and again cooled to 5 deg C.
Thereafter, a solution of 0.5 gram sodium nitrite in 4 ml of water was added dropwise while stirring over the course of about 5 minutes. Crystal
growth was much better using this method, forming a fine gllistening product over the course of about 10 minutes. It's still drying though. 

[Edited on 26-6-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by nitro-genes  | Great finds Rosco! Initiating power equal to lead azide for the potassium salt of DDNR sounds great, looks like it can be recrystallized from water as
well. Difficult part will undoublly obtaining the dinitro aminorescorcinol, and SnCl2 to perform the reduction.
The fun with these compounds just doesn't seem to stop. Apart from the fact that paracetamol makes an easy route to isopicramic acid and a word class
primary(ies?), the isopicramic acid also seems to crystallize as an almost pure gold compound...and burning it is very funky. Burning one gram
reminded me somewhat of mercury(II)thiocyanate, producing these organic looking "branches" of carbon when it is burning. Picture attached, it was even
better looking before I tried to move it and part of it fell apart. 
Oh, also did an experiment with diazotization in ethanol by the following procedure:
1 gram of recrystallized isopicramic acid was dissolved in 50 ml of ethanol (gentle heat applied to speed things up). This was placed in an ice bath
and allowed to cool to about 5 deg C again, after which 1 ml of 98% sulfuric acid was added (no sign of precipitation) and again cooled to 5 deg C.
Thereafter, a solution of 0.5 gram sodium nitrite in 4 ml of water was added dropwise while stirring over the course of about 5 minutes. Crystal
growth was much better using this method, forming a fine gllistening product over the course of about 10 minutes. It's still drying though.  |
Great news that the alcohol idea is working out. There are several variations using alcohol with or without acetic acid and in different orders of
addition for different nitrous precursor reagents to accomplish the diazotization, so I figured one or more of the alcohol processes should work to
provide a complete diazotization and decent crystals. Technique is important with difficult solubility of the isopicramic acid being the complication
to overcome.
About the DDNR it isn't necessary at all to start from Styphnic Acid and make styphnamic acid by reduction and diazotize that. DDNR is the same
material as compound [8] of the Klapotke article and is the same compound as the Von Herz patent and is the same compound as the circa 1881 JCS
article, and is the same compound as I am identifying can be made from paracetamol first being made into a 3-nitroparacetamol, and further nitrated to
the 2,3,6 trinitroparacetamol, deacetylated, and diazotized by simply heating with azeotropic HNO3 as did Klapotke. What occurs is that one of the 3
nitro groups is hydrolyzed and decomposed to a hydroxyl during the heating with HNO3 which supplies the NO2 that accomplishes the diazotization, and
ends with DDNR. DDNR and compound [8] are identical compounds produced by different reaction schemes.
I have identified the scheme for conversion of paracetamol to compound [8] whose potassium salt is identicval with the potassium salt of the Von Herz
and later patent also and
identical with the potassium salt in the 1881 JCS article.
They are all 3 or more descriptioins of the same compound,
which can also be made from paracetamol. 
There are some later patents which reference the potassium salt of DDNR as having danger during crystallization from synthesis and handling danger and
being sensitive, reporting that accidental explosions of the potassium salt have occurred, but no details are given. See patent US6946042 attached
column 3 line 5. The patent refers to such compounds as "diazinates" The patents referenced are the German language versions of the same patents
attached in the preceding post.
Attachment: US6946042 Pyrotechnic Body.pdf (597kB)
This file has been downloaded 744 times
Thanks to Boffis for the original circa 1881 German language article for which an English language abstract is attached and a screenshot posted above.
This Benedikt and Hubl article from 1881 is the original work regarding styphnamic acid and its diazo-oxide or quinone diazide energetic derivative
which is analogous to picramic acid and its derivative DDNP, and to the isopicramic acid of Dabney and its derivative iso-DDNP or p-DDNP.
The trivial terms like DDNP for the phenol derivative or DDNR for the resorcinol derivative are somewhat inaccurate abbreviated names for these
compounds which actually lose a hydrogen from the 1 position hydroxyl and are anhydrides. This is why the same compounds have a confusing assortment
of names that are synonymous because of no single convention for naming, and reverting to the benzene itself and then assigning the 1 position for the
Oxygen associated anhydride, the compound is called a benzene diazo-oxide which is probably the most correct, but the trivial name association is lost
with what trivial phenol, or resorcinol, or other type phenol is the parent compound most associated with the derivative. So the trivial names like
DDNP or DDNR are not exactly correct for the diazo derivatives which are anhydrides, so I assigned a name that seemed most logical to me for the
potassium salt which even Von Herz was awkwardly naming different ways.
potassium 4-diazo-2-6-dinitro-resorcinol-(1-anhydride)
The later patents will refer to the DDNR itself as diazine but diazide could be used instead and neither are really correct but are abbreviations on
the term quinone diazide which is not entirely descriptive either. And the patents may refer to the salts of DDNR as diazinates which is a
nomenclature that makes even less sense to me, because it is not the diazo group which is acidic and forms a salt, but is rather the resorcinol (or
other phenolic) hydroxyl. A potassium salt is therefore a diazodinitro-anhydride-resorcinolate which is too much of a mouthful to say and almost as
awkward to type or write  So the best I can do with naming this compound
associated with the recognized trivial name parent compound resorcinol, for the potassium salt is So the best I can do with naming this compound
associated with the recognized trivial name parent compound resorcinol, for the potassium salt is
(3-potassium)-4-diazo-2-6-dinitro-resorcinol-(1-anhydride)
DDNR seems like a winner for the trivial name about as good as DDNP for the monophenol but it should be understood that through the literature there
are going to be an assortment of names encountered for the exact same compound DDNR and likewise will be the case for other diazo nitrated
polyphenols.
So far as the IUPAC convention for nomenclature well, Klapotke tossed it aside by designating compound [8] which is DDNR
as 6-Diazo-3-hydroxy-2,4-dinitrophenol when it is just about guaranteed the 1 position hydroxyl is only an Oxygen and this is an anhydride similarly
as is DDNP. Therefore, more correctly
if a name applies to associate with a conventional name parent phenolic compound I think for most exactness it would be
4-diazo-2-6-dinitro-resorcinol-(1-anhydride)
or maybe that's just me  I haven't yet checked the analysis to confirm the
math, is it 1 Oxygen and no Hydrogen actually there on that alleged "phenol" ??.....anybody else ???? I haven't yet checked the analysis to confirm the
math, is it 1 Oxygen and no Hydrogen actually there on that alleged "phenol" ??.....anybody else ???? 
Don't count your Hydrogens before they are hatched. 
See attached
Über Dinitro- und Trinitroresorcin
R. Benedikt, A. Freih v. Hübl
Monatshefte für Chemie und verwandte Teile anderer Wissenschaften
Volume 2, Issue 1 , pp 323-330
Attachment: Di und Trinitroresorcin Montsch f chem 1881 Benedikt & Hubl p323.pdf (164kB)
This file has been downloaded 581 times
With regards to the DDNR and its potassium salt, there is a possibility a double salt with nickel styphnate could form that may have interesting
properties. A double salt is reported for ordinary potassium styphnate with nickel styphnate, and it is unknown if likewise a similar double salt
may form with the potassium salt of DDNR but this possibility should be tested.
Another possibility that seems likely is that DDNR may form basic salts such as a basic lead DDNR salt, and these may form double salts with the
normal salt, resulting in a hemi-basic double salt. Also it is a possibility that a basic salt of DDNR such as basic lead DDNR could form a neutral
double salt, a bridged double salt could form, for example a picrate if the basic lead DDNR salt was neutralized with picric acid, a neutral lead
picrate / lead DDNR double salt may result. The same scheme may follow for other basic DDNR salts which could form neutral double DDNR / picrate
salts.
If the basic DDNR salt was treated instead with styphnic acid it is possible a neutral bridged double salt could form from 2 of the basic DDNR salt
molecules with styphnic acid, for example a double salt in the case of the lead salt, where 2 basic lead DDNR molecules would be neutralized by 1
styphnic acid to form a complex salt. The same scheme may hold true for other basic salts. For example a nickel complex salt or a strontium complex
salt might follow such a scheme where a pair of basic salt DDNR molecules is converted to a neutral complex salt by adding to 1 styphnic acid
analogous as adding to 2 picric acids, as the 1:1 scheme first described to form a neutral multiple salt.
Likewise other acidic energetic materials like the tetrazoles may form unique complex salts with basic salts of DDNR.
For any of the schemes described it is also possible the free acid DDNR may likewise react with a basic salt of the other compound such as the basic
picrate or basic styphnate, ect. to form a neutral complex salt, with only the order of addition being different.
With regards to the contemplated basic lead salts of DDNR there is also the possibility that such basic salts may form clathrates in like fashion as
does basic lead picrate. If this is indeed the case then such clathrates would be expected to have energy greater than the already known clathrates
derived from basic lead picrate. It seems possible that basic lead DDNR could serve as a substrate for an assortment of clathrate derivatives that
would all in general have greater energy than the analogous picrate based clathrates.
[Edited on 6/27/2015 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I believe I have just found another structure identification error in the early online preview article by Klapotke which is incorrectly identifying
compound [8] as a 6-diazo instead of the 4-diazo ...which could only occur if there was an improbable rearrangement. I am reasonably sure that the
compound [8] of the Klapotke article which is being designated as 6-Diazo-3-hydroxy-2,4-dinitrophenol for compound [8] is probably incorrect.
Following the same style nomenclature it would correctly be identified as 4-diazo-3-hydroxy-2,6-dinitrophenol (if it actually was a phenol) but is
likely a diazo-oxide.
Likewise I believe Von Herz incorrectly identifies the patent compound of GB207563 as being a 6-diazo or a 2-diazo
I was both right and wrong subsequently in my post just above saying compound [8] is identical to the Von Herz patent compound The patent compound of
Von Herz is identified by Von Herz the 6-diazo or 2-diazo. They are indeed likely the same compound but are incorrectly identified the same way by
both Von Herz and Klapotke!
I know how it sounds that I believe Von Herz AND Klapotke are both incorrect. There it is. I said it anyway. 
My personal belief, opinion, and assertion is that ALL of these compounds, the compound of Benedikt and Hubl described in 1881, the compound of Von
Herz described in 1922 in GB207563, the compound of Hagel and Redecker in US4246052 from diazotization of styphnamic acid, AND the compound [8] of the
Klapotke are all probably identical and are one and the same compound 
4-diazo-2,6-dinitroresorcinol-(1-anhydride)
Ha! What are the odds! Anybody want to bet on it ??
I bet the X-ray and NMR people are getting busy about right now! The whole spectrographic analysis routine is going to be needed, infra red analysis
included. 
It will be interesting to see where this goes from here.
The article by Klapotke which is being referenced is attached in an earlier post
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
attached again here
http://www.sciencemadness.org/talk/files.php?pid=409043&...
and the web page for the article preview is here
http://onlinelibrary.wiley.com/doi/10.1002/ejoc.201500465/ab...
To be specific, compound [7] is incorrectly identified in the abstract but correctly identified in the article itself
Also I believe compound [8] is incorrectly identified in both the abstract and the article as the 6-diazo when it should be the 4-diazo
The word description does not reconcile with the diagrams, however the diagrams are showing structure correct for [7] but show a transposing of the
anhydride Oxygen and phenol hydroxyl for [8]
The structural diagram in the article is correct for compound [7] however (not correct) for [8]. The diagrams are inverted from what is the usual
view. Additionally the anhydride Oxygen and the phenol hydroxyl are transposed on the diagram shown for [8]
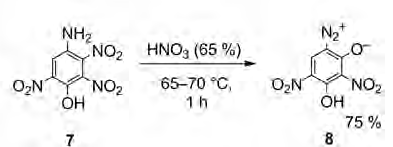
[Edited on 6/28/2015 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
That is some serious detective work you are doing there! You make a good case and it would be interesting to see some proof in the form of chemical
& structural analysis results.
[Edited on 28-6-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Exactly what I was thinking, given the "unfinished business" about structures being left in Reverdin's hands by an aging Meldola a hundred years ago
and all the published material about "mobility" of the nitro group, and NOT also mobility of the diazo group, the structural identifications remain
perplexing and even an enigma. The older literature is confusing to follow as it would seem there definitely are 2,3,5 and 2,3,6 trinitro compounds
and that there would be differing reactivity for them. Changes in nomenclature and synonymous but different names being used including different
conventional and different informal common names have added to the confusion.
Reviewing the different methods of synthesis using modern analytical tools as well as the classical chemical analysis such as doing mixed melting
points and other mixed sample comparisons will surely remove the uncertainties that are otherwise unanswered questions about the actual
identifications of all these compounds which is not presently conclusively known, from what I see.
I have certainly set a needed synthesis and analysis survey task before the chemists who are looking at this.
When looking first at iso-DDNP which we are identifying as p-DDNP, it had been noticed while reviewing the literature that this was leading to many
other related interesting energetic compounds that in my opinion are not really settled business in the more than one hundred years since their
discovery.
There should be a burning of the midnight oil occurring at a few different laboratories regarding this subject matter.
When I first started looking at this subject matter I had the feeling this was going to be a journey into the tall grass of some detective work 
Some of this I have already begun to sort out as really no greater issue than a difference in nomenclature which is a departure from conventional more
usually encountered
nomenclature. There are different ways of identifying the exact same compound which are technically correct, depending upon what direction, clockwise
(conventional) or counterclockwise (unconventional) is performed the counting of the ring position for substituents and in the case where there are
two hydroxyls, which of the hydroxyls is arbitrarily selected as being ring position 1. Generally seen is resorcinol shown as a 1,3-dihydroxybenzene
however 1,5-dihydroxybenzene would also apply as would m-dihydroxybenzene be synonymous.
The usual convention for resorcinol and derivatives would be shown as the 1,3-dihydroxy configuration, going clockwise with 1 at 12 o'clock and 3 at 4
o'clock.
PHILOU Zrealone pointed out the differing but synonymous nomenclature
http://www.sciencemadness.org/talk/viewthread.php?tid=22586&...
which illustrates that for example styphnamic acid by conventional nomenclature is 4-amino-2,6-dinitroresorcinol, but in mirror image reverse
perspective for the exact same compound could be called 6-amino-2,4-dinitroresorcinol and these are not isomers but are simply different nomenclature
being used to describe exactly the same compound.
Given the example by PHILOU Zrealone then the inconsistency about nomenclature could help explain the designation by Klapotke of compound [8] as a
6-diazo-2,4-dinitro gotten from a different nomenclature identified precursor which was called a 4-amino-2,3,6-trinitro. At a reach this is
technically correct but it is confusing to change from one nomenclature to another that is a mirror reversal perspective done without any explanation
or notation as changing horses in midstream while diagramming a reaction sequence. And the anhydride Oxygen and the phenol hydroxyl are still (I
think) transposed, so the nomenclature is an awkward expression.
None of the departures or variations in nomenclature change my opinion that all of the various different descriptions are each in their own special
way identifying the same compound.
Anyway, even the IUPAC convention suggests benzene-1,3-diol for resorcinol placing the hydroxyls at the 1,3 position.
And I think the trivial name for compound [8] would be DDNR
analogous to DDNP. I still think the nomenclature I suggested
4-diazo-2,6-dinitroresorcinol-(1-anhydride)
is most exacting, or for the alternative hydroxyl location for the anhydride Oxygen if that proves to be the case
4-diazo-2,6-dinitroresorcinol-(3-anhydride)
Sorting through the many different nomenclature designations for what appears likely the same compound has been confusing for me.
[Edited on 6/29/2015 by Rosco Bodine]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
isopicramic acid perchlorate
Quoted from PM from Rosco Bodine 6-6-2015 at 00:48
| Quote: |
What do you think about that possibility that a low solubility isopicramic acid perchlorate may exist?
Do you think the formation of a precipitated salt would be unlikely from mixing solutions of isopicramic acid sulfate and magnesium perchlorate?
If such an isopicramic acid perchlorate does exist, it could be a primary explosive since isopicramic acid is itself explosive.
If the isopicramic acid perchlorate does exist, unless it is corrosive and hygroscopic, it could possibly have interesting properties as an initiator.
If so, it could possibly be used with other perchlorate salt base charges like guanidine perchlorate or methylamine perchlorate, or trimethylamine
perchlorate, or tetramethylammonium perchlorate. I am thinking that isopicramic acid perchlorate could be similar to triaminoguandine perchlorate and
would be easier to produce. |
I reply to this here and not in PM because it is of interest for most readers of this tread and of energetic materials...so better keep this public
than private  , I'm sure Rosco will agree with me , I'm sure Rosco will agree with me  on that. on that.
Picramic and isopicramic acid does display amphoteric properties owing to the simultaneous presence of both amino and phenolic groups on the aromatic
ring, but acidity of those is increased by the presence of the two nitro groups, of the hydroxy and of the amino groups.
More than certainly there is a zwiterionic effect
NH2-Ar(NO2)2-OH <--> NH3(+)-Ar(NO2)2-O(-)
And this may lead to solubility troubles in neutral media but will ensure good solubility in strong basic or in strong acidic media.
The idea to play with perchloric acid is a very good one!
1°)Because picramic acids are initially not wel oxygen balanced and the introduction of one HClO4 is like having two extra nitro groups into the
molecule...
HClO4 --> HCl + 2 O2
2 -NO2 --> N2 + 2 O2
Thus the OB is wel enhanced.
2°)Perchloric acid is very strong acid and thus it will allow formation of a perchlorate even if picramic acid is acidic.
3°)Perchlorate ensure good sensitivity to initiation and crystallinity
4°)Perchlorate ensure good density...it will boost up the density of picramic acid by a few %.
All this will make the resulting picramic perchlorates interesting compounds on their own with regard to brisance and VOD...
HO-C6H2(NO2)2-NH3ClO4
Sadly one can't get reaction with a base from the phenolic part because it will decompose the amine perchlorate ...the base will react with the
stronger acid...what is HClO4...
The result will be a mix of picramic acid (or picramate) and metalic perchlorate salt...what will be interesting but much less than the plain picramic
perchlorate.
The procedure you propose should work, but MgSO4 is quite soluble...see Epsom salt.
I would go for direct "neutralisation" of 1 equivalent of picramic acid and 1 equivalent of HClO4...then if no precipitation...evaporation in the cold
or mild heat and if needed with help of vacuum.
Or alternatively... Picramic sulfate with saturated Ba(ClO4)2 but the resulting solution must be filtered for BaSO4.xH2O and Ba perchlorate is harder
to get than plain HClO4...
Corrosivity and hygroscopicity of the compound is not a real concern... because now everybody can work cheap with PE plastic recipient or staws and
thermic glue that will be entraped on site into the detonator (I work that way to prevent contact between reactive chemical explosive - personal
safety concern due to some bad experiences).
Note that another good idea would be to make the nitroformiate
--> picramic acid trinitromethanate
HO-C6H2(NO2)2-NH3C(NO2)3
If the perchlorate is very good maybe it would be good to invetigate the periodate (owing to the density boost)
--> picramic acid periodate
HO-C6H2(NO2)2-NH3IO4
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Since that U2U message was sent I have thought more on that type of metathesis approach to avoid use of HClO4. It may work also to use the soluble
isopicramic acid hydrochloride and dissolve into the acidic solution ammonium perchlorate. It may work similarly for the isopicramic acid sulfate.
If the perchlorate of isopicramic acid does form a stable salt and shows low solubility it should precipitate without requiring HClO4.
Thanks for the feedback on the perchloric acid idea regarding isopicramic acid. The use of perchloric acid may also be interesting in regards to a
possible styphnamic acid perchlorate salt.
A related idea is perchloric acid could react in the synthesis leading to DDNR which is believed to form via a soluble diazonium salt intermediate but
the diazonium salt is never isolated and quickly decomposes to form the diazo oxide. Likewise a similar thing may occur during the formation of DDNP
or p-DDNP. So an idea I had is that if the intermediate diazonium salt, formed as a perchlorate and precipitated during diazotization, instead of
decomposing to the diazo oxide, such a diazomium perchlorate could have interesting explosive properties. But I have found nothing in the literature
ever mentioning even any attempt to isolate the theoretical intermediate diazonium salt which is unstable and transitions quickly to the diazo oxide.
I am just about 100% certain that the compound DDNR and/or its potassium salt reported by various methods of synthesis by different researchers
beginning with Bemedikt and Hubl circa 1881, Meldola in 1906, Von Herz in 1922, Hagel and Redecker 1981, and Klapotke 2015 are describing exactly the
same compound as I gave this name which seemed to fit best.
4-diazo-2,6-dinitroresorcinol-(1-anhydride)
or for the alternative hydroxyl location for the anhydride Oxygen if that proves to be the case
4-diazo-2,6-dinitroresorcinol-(3-anhydride)
The curious thing to me is that there has been no history of citations from the latest article going back to the earliest. None of the 4 reporters of
the same compound seem to be aware of the previous original work, which I suppose could happen for an obscure compound. I chuckle at the thought the
same compound has now been invented and discovered maybe? 5 times over the course of 134 years  maybe just because the compound was never satisfied it had been named correctly maybe just because the compound was never satisfied it had been named correctly 
I have in general set forth how I think it is possible to produce DDNR starting from ordinary paracetamol in earlier posts. I have not yet set forth
with good clarity the step by step which depends upon sorting out carefully some of the articles by Meldola who worked with the necessary intermediate
which is a trinitro derivative of paracetamol that cannot be gotten from direct nitration in one step of paracetamol. Two nitration steps are
required starting from paracetamol to avoid need for use of acetic anhydride. I have been carefully rereading the Meldola articles after learning the
initial discovery and early work done on the first known trinitroacetylaminophenol had mistakenly identified which isomer was being described in
publication. This had been my suspicion and a source of frustration before accessing the later articles that identified and confirmed the errata. So
a rereading of the early Meldola articles about the first identified 2,3,5 trinitroacetylaminophenol is required with a mental note being done that
it is actually the 2,3,6 isomer which is being described and the chemistry and properties and observations are otherwise valid regarding the subject
matter notwithstanding the early work showing a mistaken identification of the isomer.
Here is an example of the sorting out of the information from Meldola, noting that the diazo-oxide later obtained from boiling with sodium acetate the
initial diazotization product of 2,3,6-trinitro-4amino-phenol is one and the same compound as compound [8] now described by Klapotke and evidently
also 3 times before identified by others probably unaware of the prior art of Benedikt and Hubl since none cite that original work.
The initial diazotization product of 2,3,6,-trinitro-4-aminophenol shows the 3-nitro group remaining intact, and only becomes DDNR via subsequent
decomposition of the first obtained 2,3,6-trinitro-4-diazo-phenol-(1-anhydride) which may be even more energetic than DDNR. If the the boiling with
initial diazo-oxide having the 3-nitro intact was performed using potassium acetate instead of sodium acetate it is probable the potassium salt of
DDNR would be the result, which is likewise the potassium salt of the compound [8] of Klapotke.
A warning note should be made regarding the observation of Meldola that the trinitroacetylaminophenol is truly extremely reactive and unstable and
cannot be dried in contact with filter paper even at low heat, because it will deflagrate. It must be dried only in contact with glass or porcelain.
This first image shows the isomer misidentified

Updated data for the convenient deacetylation is also provided later, here. Also we see another description of the second diazo-oxide derivative
identified which is one and the same as compound [8] of Klapotke, which is the compound Meldola names dinitrohydroxyquinonediazide.
 
Note that all of the 2,3,5 trinitro references above and below are incorrect and actually are the 2,3,6 trinitro compound.
  
This next image shows the later structure correction data for the same compound/(s) above as earlier designated 2,3,5 trinitro was later amended /
corrected by the authors to 2,3,6 trinitro
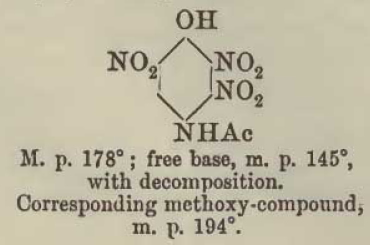
What this data suggests is that it may be possible to produce DDNR from a modified deacetylation procedure applied to the trinitroacetylaminophenol,
which upon deacetylation is subject to self-diazotize from decomposition of the position 3-nitro group. Suggested also is the possibility that active
diazotization may be possible to be applied directly to the trinitroacetylaminophenol which may be simultaneously deacetylated and diazotized leading
to the desired DDNR, by a process which is a shortcut and may be workable to avoid the isolation of the intermediates by separate steps of
deacetylation followed by diazotization. It is unknown if such a shortcut is possible or not or what would be the yield. It may work or may not work
to attempt such elimination of the isolation of the intermediates, and combine the reactions in a one-pot kind of reaction scheme.
Attachment: article Meldola and Hay JCS Vol95 pg1378.pdf (1MB)
This file has been downloaded 551 times
[Edited on 6/30/2015 by Rosco Bodine]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
In old books a typo very fast occurs and is not noticed for long...
So initially the compound was indeed 2,3,6-trinitro-4-acetamido-phenol and not 2,3,5-trinitro-4-acetamido-phenol.
Since the initial nomenclature name was wrong probably that nobody has noticed this error nor the earlier finding in the litterature...
I have re-reread an old book from 1948:
Traité de Chimie Organique, by V. Grignard, G. Dupont, R. Locquin (+ Paul Baud)
Tome XV: Diazoïques et azoïques - Triazènes, Tétrazènes et Pentazdiènes - Azoxydérivés - Hydrazines - Hydroxylamines - Oximes -Amidoximes -
Azides
Editor Masson & Cie
In the alcanic and aromatic series ... very interesting chemistry.
I have found a lot of valuable infos, but there are also some discrepencies and errors (typos)... sometimes the more you read, the more you have to
read again and say to yourself...wait a minute.
Diazoniums salts are really very hard subject.
I will come back shortly with a lot of comments about this for this tread!
[Edited on 30-6-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Beta Isopicramic Acid and Beta p-DDNP
Earlier in the discussion of p-DDNP and its isopicramic acid precursor I made the observation there is yet another para diazo isomer of the commonly
known ortho diazo DDNP, so that in fact there exists the Alpha isomer p-DDNP which is gotten from the isopicramic acid of Dabney, but also there is a
Beta isomer p-DDNP obtainable from a Beta Isopicramic Acid identified by Meldola. It is expected that stability for the Beta p-DDNP will be the lowest
of the 3 isomers of DDNP but to what extent or what bearing this may have on the explosive power and sensitivity is unknown. No literature reference
is found regarding the Beta p-DDNP isomer to provide any further information.
Alpha p-DDNP is the 2,6-dinitro-4-diazophenol-(1-anhydride)
Beta p-DDNP is the 2,3-dinitro-4-diazophenol-(1-anhydride)
In earlier mention of this Beta p-DDNP isomer it was not wished to derail the topic while attention was focused upon the Alpha p-DDNP isomer, so
further mention of the Beta p-DDNP isomer has been deliberately delayed until now.
It seems the appropriate time to point to this Beta p-DDNP and its associated Beta Isopicramic Acid precursor.
Structurally this is the Beta Isopicramic Acid of Meldola
And below is the Beta p-DDNP from diazotization of the Beta Isopicramic Acid
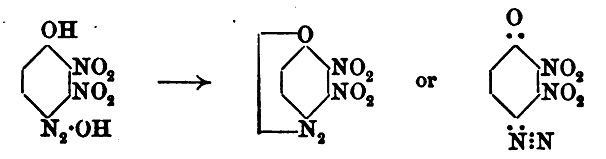
A screenshot from the Meldola and Hay article attached below the screenshot provides additional information for which a caveat must be applied to any
reference to the 2,3,5 identified trinitro compounds which were being misidentified at the time of the article. The information is otherwise valid,
that caveat regarding the misidentification of a third nitro group position notwithstanding. The structure error for that aspect has no bearing on
the subject matter of the Beta Isopicramic Acid and the Beta p-DDNP product of its diazotization.

The red crystalline product from deacetylation described below is the Beta Isopicramic Acid which is also reportedly unstable, so to optimize the
yield of Beta p-DDNP it would probably be best to proceed with diazotization of the sulfuric acid solution diluted to the extent it may withstand
dilution, without attempt to isolate the free Beta Isopicramic Acid. There will be expected losses from decomposition during every manipulation of the
unstable Beta Isopicramic Acid.
 
Attachment: Beta Isopicramic Acid and Beta p-DDNP JCS_London Vol 91 Meldola and Hay pg1477 and 1481.pdf (427kB)
This file has been downloaded 590 times
Of course the door is opened to BOTH the Beta Isopicramic Acid and Beta p-DDNP as well as the synthetic route to DDNR by the availability of the
3-nitroacetaminophen reported obtained in 81% yield from a nitrite nitration scheme applied to paracetamol, this being reported by the Japanese
pharmaceutical bulletin which I believe is reliable. This avoids having to work with acetic anhydride to first add an O-acetyl to paracetamol to form
the diacetylaminophenol and then nitrate and then deacetylate and then nitrate some more, and then deacetylate again, prior to diazotization. It
really saves steps in synthesis and simplifies things greatly to have the 3-nitroacetaminophenol easily available in high yield directly from
paracetamol. That is the key to simplifying the synthetic routes and processes described in the literature before, including the most recent article
of Klapotke.
So this is breakthrough type work we are discussing here.
Attachment: A NEW NITRATION PRODUCT, 3-NITRO-4-ACETAMIDOPHENOL, OBTAINED FROM ACETAMINOPHEN WITH NITROUS ACID.pdf (178kB)
This file has been downloaded 521 times
I apologize for the somewhat rambling style of posting my thoughts concerning these old references which I have been trying to sort out and understand
for the past year as my available time has allowed. I think in these last two posts I have completed the analysis of the available references in a
way that connects the dots and makes sense.
There likely is a series of actual 2,3,5 trinitro compounds and chemistry that is analogous to the 2,3,6 trinitro compounds, and other related
compounds based on phloroglucinol or other polyphenols. I have not looked at those so far and have tried first to sort out the 2,3,6 trinitro series
since it appears to be the logical first step, before looking at the likely more difficult variants. It was the common paracetamol being a precursor
which made the 2,6 and 2,3,6 series of compounds especially interesting.
[Edited on 6/30/2015 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Regarding preparation of the 3-nitro-4-acetylaminophenol which is the precursor that would make convenient synthesis of DDNR and the associated
2,3,6-trinitro-4-diazophenol-(1-anhydride) precursor which is also of interest:
There is a contemplated synthetic approach based upon the Japanese Chemical and Pharmaceutical Bulletin article which I have posted as a proposed
experimental in another thread
http://www.sciencemadness.org/talk/viewthread.php?tid=9722&a...
|
|
|
| Pages:
1
..
17
18
19
20
21
..
33 |
|