chem_haruka
Harmless

Posts: 6
Registered: 8-3-2015
Member Is Offline
Mood: No Mood
|
|
why HO2 is a stronger acid than H2O2
pKa(HO2)=4.88 pKa(H2O2)=11.8 pKa(HO)=11.9 pKa(H2O)=14(15.6)
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
What is "HO2" and where can I get some?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
exactly what i thought when i saw this
a quick google search gives
http://en.wikipedia.org/wiki/Hydroperoxyl
>The hydroperoxyl radical, also known as the perhydroxyl radical, is the protonated form of superoxide with the chemical formula HO2
i would think that HO2 is more acidic because it's a free radical as opposed to H2O2 which is much more stable
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quoth Wikipeda
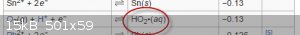
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Ah, a radical. That explains it, then. And yeah, a radical is usually much more reactive than a compound.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
HO2 is very unstable and only exists for a very short time. Salts of this acid, however, are quite stable and the potassium salt (KO2) can be
purchased commercially. This salt is orange. When this salt is added to water, then the acid HO2 is formed, together with KOH, and the acid HO2 then
quickly decomposes, giving O2, H2O and some H2O2.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
https://www.youtube.com/watch?v=c-wjagDxx68
http://en.wikipedia.org/wiki/Potassium_superoxide
http://www.sigmaaldrich.com/catalog/product/aldrich/278904
Never heard of this. Learn something new every day!
(Thanks woelen.)
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
How's about OHO !!  
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
OHO OHO OHO
Oxidising Santa.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
santa doesnt like this
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
Actually no, because the deprotonated form (superoxide) is a radical as well, so nothing is gained in terms of stability by deprotonation on that
front.
I think the reason for the increased acidity of HO2 compared to H2O2 has to do with the fact that the bond order of the O-O bond in H2O2 is 1, whereas
in superoxide it is 1.5. This means that p orbitals are being used up to create a pi bond between the oxygens, and as such the hybridisation of the
oxygens will be that of less p character and more s character. As s orbitals are on average closer to the nucleus than p orbitals, increased s
character means that the electrons will be held onto more tightly, and less available for bonding. As such, any proton bound to the oxygen will have a
weaker bond and be more easily removed. This is the same phenomenon that makes alkynes stronger acids than alkenes, which are in turn stronger acids
than alkanes.
Never accept that which can be changed.
|
|
|
DrMario
Hazard to Others
  
Posts: 332
Registered: 22-9-2014
Member Is Offline
Mood: Underpaid.
|
|
Well played, sir. And thank you, because your post generated this beautiful reply, very much worth quoting:
Quote: Originally posted by Pyrovus  |
Actually no, because the deprotonated form (superoxide) is a radical as well, so nothing is gained in terms of stability by deprotonation on that
front.
I think the reason for the increased acidity of HO2 compared to H2O2 has to do with the fact that the bond order of the O-O bond in H2O2 is 1, whereas
in superoxide it is 1.5. This means that p orbitals are being used up to create a pi bond between the oxygens, and as such the hybridisation of the
oxygens will be that of less p character and more s character. As s orbitals are on average closer to the nucleus than p orbitals, increased s
character means that the electrons will be held onto more tightly, and less available for bonding. As such, any proton bound to the oxygen will have a
weaker bond and be more easily removed. This is the same phenomenon that makes alkynes stronger acids than alkenes, which are in turn stronger acids
than alkanes. |
|
|
|