dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
Bromo-methane Disposal - Quick Question
Quick Question, gents.
Just routinely demethylating an anisole to produce a phenol. More specifically, 1-methoxy-4-phenylmethan-N-N-propan-8-olmethylamine.
I hope I got that correct, lol.
How do I deal with the bromo-methane that comes out of the reaction? I don't really want to be responsible for depleting a small amount of the ozone
layer. I know I won't be on the same level as Thomas Midgley Jr. but it'd be nice to not release environmentally dangerous gases - even if they are
absorbed by the charcoal i use in my fume hood.
Can I just bubble it into a vat of sodium hydroxide? If not, how should I render the MeBr safe?
A side question - I was going to venture this and see my end results, but since i'm here anyways, might as well ask:
Would the nitrogen at the 6th position be affected by the usage of HBr? I can see a potential demethylation of the tert-amine but I think the
oxygen would be the primary product - would that be the case? The A-B reaction should be quicker... But I'm not sure. I have no experience with
demethylation of amines - not even sure it will happen.
My entire quarrel with pyridinium HCl is that I'd have to run a column. Considering this is an intermediate step to making something else, I really
don't want to run a column for this. Would that be easier as well?
[Edited on 22-4-2015 by dermolotov]
[Edited on 22-4-2015 by dermolotov]
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
MeBr + NaOAc -> MeOAc + NaBr
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
unless you're doing large scale manufacture of something, releasing a little gaseous MeBr isnt going to hurt anyone or anything.
This gas is dangerous to the environment in tonnage quantities, not grams.
Otherwise you'd probably need to treat it with an alkali hydroxide or a methoxide compound in aq conditions underpressure to convert it to methanol?
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by Templar  | unless you're doing large scale manufacture of something, releasing a little gaseous MeBr isnt going to hurt anyone or anything.
This gas is dangerous to the environment in tonnage quantities, not grams.
Otherwise you'd probably need to treat it with an alkali hydroxide or a methoxide compound in aq conditions underpressure to convert it to methanol?
|
I agree, a small amount of methyl bromide isn't going to be a big deal as long as you don't live on top of your neighbors. As for dealing with it if
you still want to neutralize it, a solution of sodium or potassium hydroxide should react with it to form sodium bromide and methanol. And a sodium
acetate solution is not going to work very well, acetate is a not a great nucleophile. Reaction with methoxide will be a Williamson ether synthesis
and you will get dimethyl ether which may be a pain in it's own regard, but is still less toxic than methyl bromide.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
I aided in the release of 68 pounds of methyl bromide (in the gas phase) last Saturday as a fumigant.
It is a viable, and in a few cases the only fumigant which will work on some things.
Do not breath it, do not get it on your skin if it it is cool enough to be liquid, probably not wise to get it on your skin in the gas phase either.
It is still being used big time. The "Montreal Protocol" has guidelines for its reduction, but it will be a long time before its use gets small.
The amount you will make is not going to change the environment but use caution to make your exposure small.
It can kill the small as well as the large.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The simplest way to fix this is to dissolve it in some sort of cold nonpolar solvent (MeBr boils at 5º C) and react with magnesium. To ensure a fast
reaction you can put the magnesium in the solvent and then scratch the surface so there's no oxide layer. MeBr reacts with Mg in seconds.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
It is not very practical to trap small quantities of MeBr. I have not seen any lab trap that type of compound, just vent it well. On a lager scale,
it would be trapped in a cold trap and then disposed off. But NaOH in MeOH might work, acetone/K2CO3/nucleophile would likely work, be careful to
prevent suck back in the event of a change in pressure. I was also going to mention that it is used in ton quantities to fumigate wheat and other
crops to kill bugs. Not a great thing for the environment, but one of the many tools to allow us to feed 8 billion people. So the solution to
pollution is dilution, or better yet, a lower population.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Although MeBr is a toxin, it is pretty innoculous as such things go. If you use a cold trap to condense it and then react it in an ice cold sodium
hydroxide, you should be able to destroy most of it and vent the rest. My only concern would be good ventilation as the LC is about
1.4g/M3.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Guys, was I writing to myself?
Halogenated methane derrivatives, such as methyl chloride, methyl bromide, chloroform, dichloromethane, methyl bromide, dibromomethane, etc., are
really stable and hard to hydrolyse.
Rate constant for MeBr + NaOH reaction is something like k=0.02 (r = k*[MeBr]*[NaOH]). This means that for 1N solution of NaOH you will get reacted
0.02 moles of MeBr per second per mole of MeBr.
Actually alkali solution is used for purification of MeBr:
https://archive.org/stream/bromideasi00unse/bromideasi00unse...
"Preparation of methyl bromide. Jour. f. Prakt. Chcm. 104: 285-288. 1922. [in Gorman.]
Sulfuric acid (95 percent) is first diluted with water, the methyl alcohol is slowly added with cooling, followed by pulverized potassium bromide, and
the flask with reflux condenser is slightly warmed. The evolved gases arc passed through water or sodium hydroxide to remove hydrobromic acid and then
through two bottles of concentrated sulfuric acid. Methyl bromide is then condensed in an ioc-sali bath. The yield varied between 93,5 and 97.3
percent, depending on the proportion of methyl alcohol to the other reactants."
So just take a goddamn sodium acetate. You migh also add some base to it because sodium acetate is only weakly basic and might not react as fast as
needed.
Also, you might try ammonia solution, though it's too volatile.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Yes, it will react slowly. This is a non-zero conversion rate as anyone who has left chloroform overnight with bleach solution will tell you. And
having to maintain it close to 0C is also an issue. Sodium acetate may be faster but most chemist have sodium hydroxide on hand. Not that you
couldn't just mix some vinegar and baking soda and wait until it gets syrupy. Of course you aren't going to want to use 1N, more like 10M sodium
hydroxide which is close to saturated at 0C. You don't even need pure sodium hydroxide, drano should work fine. And that is OTC while sodium acetate
requires work.
What is the reaction rate with sodium acetate anyway?
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Methyl bromide and methyl chloride are rarely used nowadays, so I managed to obtain only methyl iodide data.
https://www.jstage.jst.go.jp/article/jcej1968/7/5/7_5_364/_a...
This article mentiones something like k1=0.03 (1/h) [OH-] independent constant and k2 = 0.75 (L/mol/h) for NaOH hydrolysis:
r = ( k1+k2*[OH-] ) * [CH3I] (in fact it is ([CH3I] - [I-]) but we suppose that there's no halogen salts in the solution).
Remember that I mentioned k2=0.02 (L/mol/h) for MeBr hydrolysis in my previous post. Iodine is a better leaving group, this is why MeI reacts faster.
And this article mentiones phenol alkylation with MeI in DMF using TMGN as a base:
https://www.usc.es/congresos/ecsoc/13/hall_a_GOS/a39.pdf
They say about k=10 (L/m/h), r = k*[PhO-]*[MeI], which is at least 10 times higher than MeI hydrolysis in water with NaOH.
I'm not really sure if you can directly compare this stuff, feel free to argue.
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Quote: Originally posted by byko3y  | Guys, was I writing to myself?
Halogenated methane derrivatives, such as methyl chloride, methyl bromide, chloroform, dichloromethane, methyl bromide, dibromomethane, etc., are
really stable and hard to hydrolyse.
Rate constant for MeBr + NaOH reaction is something like k=0.02 (r = k*[MeBr]*[NaOH]). This means that for 1N solution of NaOH you will get reacted
0.02 moles of MeBr per second per mole of MeBr.
Actually alkali solution is used for purification of MeBr:
https://archive.org/stream/bromideasi00unse/bromideasi00unse...
"Preparation of methyl bromide. Jour. f. Prakt. Chcm. 104: 285-288. 1922. [in Gorman.]
Sulfuric acid (95 percent) is first diluted with water, the methyl alcohol is slowly added with cooling, followed by pulverized potassium bromide, and
the flask with reflux condenser is slightly warmed. The evolved gases arc passed through water or sodium hydroxide to remove hydrobromic acid and then
through two bottles of concentrated sulfuric acid. Methyl bromide is then condensed in an ioc-sali bath. The yield varied between 93,5 and 97.3
percent, depending on the proportion of methyl alcohol to the other reactants."
So just take a goddamn sodium acetate. You migh also add some base to it because sodium acetate is only weakly basic and might not react as fast as
needed.
Also, you might try ammonia solution, though it's too volatile. |
I've run a few phosgenations that make methyl chloride as a byproduct. The methyl chloride would be captured with the phosgene laden solvent during
the strip. So then the methyl chloride / phosgene solvent would be dripped into 10C NaOH solution. Usually the phosgene would react pretty quickly
under those conditions, some would reflux off the dry ice / acetone condenser on the setup, but only an errant drip or two. But with MeCl in the mix,
the whole condenser (at 5C) would fill with a bouncing plug of MeCl which would begin to bounce off the dry ice condenser and then cool the whole
condenser until the glycol was flowing like sludge. It just did not want to react with the NaOH solution no matter what! I always thought that was
pretty neat. That was the first thing I thought about when I read this post but I thought MeBr would be more reactive than the chloride, apparently
not by much.
|
|
|
Hellafunt
Hazard to Self
 
Posts: 65
Registered: 2-12-2014
Member Is Offline
Mood: No Mood
|
|
As a total noob, I had to google Thomas Midgley Jr. after reading this thread. I read the wikipedia article about him, wow. Fascinating.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I can´t find the article right now but I think it was in New Scientist or similar about volatile methyl compounds produced by kelp beds and other
algae in the worlds oceans (there is quite a body of literatura on the subject if you check online). Apparently they produce compounds such as methyl
bromide, methyl iodide and dimethyl sulphide in quantities that exceed a million tonnes a year, I don´t recall the precise quantities but the figure
of 180,000 tonnes/year for methyl iodide sticks in my head. They article claimed that it is these compounds and not ozone that create the
characteristic small of the "sea side". It also claimed that this is the key to the mechanism of iodine concentration in the caliche nitrate deposits
of Chile (the iodomethane is decomposed by sunlight and oxygen and then precipitates from the atmosphere and accumulates in the soil since there is
Little rain to wash it away).
So dermolotov are you releasing quantities sufficient to compete with the kelp and their associated beasties? If not I shouldn´t too much. I think
the secret is just don´t let it accumulate indoors.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Lol. Gonna build my own chile to mine the iodine from the ocean.
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
The compound you indicated doesn't exist... can you post a picture please?
How are you doing this? Using HBr or via red P with Br2?
I'm interested in this method too
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Once the MeBr disposal drew my attention. It's hard to store, it's hard to dispose. It reacts sluggishly with neutral water, slightly faster with wet
charcoal, hydroxides,carboxylates, phenolates, relatively fast with aromatic amines, really fast with amines, thiosulfates (yielding Bunte salt), and
probably thiols.
http://pubs.acs.org/doi/abs/10.1021/jf00053a044 - Kinetics of hydrolysis on wet charcoal. As you can see, the temperature is important for the
reaction (and pretty much for any reaction with MeBr), methyl bromide becomes much more reactive at 40°C+.
Methyl bromide disposal is a known problem, so there are some researches on it, and also patents like US5904909 describing absorption of MeBr on charcoal and it's reaction with thiosulfate+water.
I doubt that MeBr will easily react with magnesium. It can react with aluminium, but the resulting compound is spontaneously flammable, just like
methylzinc is.
I'm thinking whether it's possible to utilize the mmethylation power the bromide to make something usefull, except methanol.
The problem with thiol/Bunte salt is that methanethiol has horrible smell. Bunte salt could be oxidized to methanesulfonyl chloride, which is a
usefull reagent, but toxic and corrosive by itself (well, at least it has strong odor). Maybe it's possible to make sodium methanethiolate, bypassing
the methanethiol, or even dimethylsulfide, which is not so horrible as methanethiol is.
Amines might be another option, but the problem is that we need a high temperature, while lower amines and ammonia are volatile and higher amines are
harder to obtain. Something like triethylamine will give triethylmethylammonium bromide.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I'm gonna post something huge and remarkable. This one answers all the questions about what reagent shoud be used for reacting with methyl bromide:
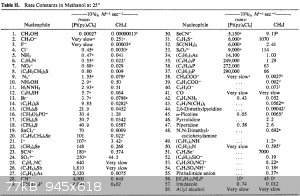
Those are valid for methanol as a solvent and methyl iodide, but I'm pretty sure the values are close to the MeBr.
The best nucleophiles ever are thiophenolate (7000 * 10^6 times greater reaction rate than with water) and phenylselenolate (x50 000 * 10^6). Then
such nucleophiles follow (in the order of decreasing nucleophility):
- thiosulfate ( NaS2O3 ), x800 * 10^6;
- trialkyl phosphine ( e.g. Et3P ), x500 * 10^6;
- hexaethylphosphoramide ( P(NEt2)3 ), x300 * 10^6;
- sulfite ( Na2SO3 ), x300 * 10^6;
- selenocyanate ( NaSeCN ), x60 * 10^6;
- tosylchloramide ( chloramine-T ), 50 * 10^6;
- iodide ( NaI ), 20 * 10^6;
- piperidine/pyrrolidine, 20 * 10^6;
- thiourea, 20 * 10^6;
- dialkyl phosphite, trialkyl arsine, triphenyl phosphine, diethylamine, 10 * 10^6.
Alkyl thiols are missing for some reason. I can estimate them to have reactivity of x100 * 10^6.
Phenolates suck ( x0.5 * 10^6 ), and carboxylates suck a lot ( x0.02 * 10^6).
|
|
|