Pasrules
Hazard to Self
 
Posts: 78
Registered: 4-1-2015
Location: Yellow Cake Deposit
Member Is Offline
Mood: Lacking an S orbital
|
|
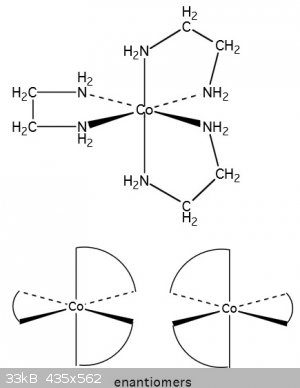
Tris(ethylenediamine)cobalt(III) chloride
Tris(ethylenediamine)cobalt(III) chloride
[Co(en)3]Cl3 . 3H2O (mw:399.625)
Quick piece of theory
Cobalt(III) complexes are much more stable than cobalt(II) complexes so by first oxidizing the cobalt a greater yield of the major product is
expected.
Chemicals:
Cobalt(II)chloride hexahydrate
30% H2O2
*30% ethylenediamine
*10M HCl
*5M HCl
95% EtOH
50% EtOH
*Diethyl ether
(* denotes kept in fumehood)
PPE:
Lab coat
Nitrile gloves
Splash goggles
Hazards:
Strong acids
Volatile chemicals
Strong oxidiser
Cobalt complex is harmful to the environment
Experimental:
Neutralise 30% ethylenediamine soln (15 cm3) with 5M hydrochloric acid (2.5 cm3). Add this soln to
cobalt(II) chloride hexahydrate (6.0g) in water (20 cm3). Oxidise the coalt(II) complex by adding 30% hydrogen peroxide (5cm3)
dropwise. Allow to stand for 10 minutes.
Transfer soln to an evaporating basin and place on a steam bath until crust forms on surface. Remove from steam bath and add 10M
hydrochloric acid (5 cm3) and stir to break up lumps. Add ethanol (15 cm3) gradually while stirring.
Once cooled filter precipitate on Buchner funnel. Close vacuum and add wash solvent then open vacuum. First solvent 50% cold ethanol, second 95%
ethanol, third diethyl ether. Place precipitate in desiccator for up to 48 hours.

Result: 57.63% yield
Notes
1. Addition of H2O2 is exothermic and may cause spitting if added too rapidly.
2. Addition of 10M HCl formed a green precipitate assumed to be a chloride complex around the edge of the basin. This precipitate was avoided when
transferring to Buckner funnel.
3. Leaving the vacuum on and pip-petting the wash solvents on dark impurities reduced product loss.
Characterization:
Column chromatography
Cation exchange (Sephadex resin)
First elution was an Orange-Red colour expected to be Bis(ethylenediamine)cobalt(III) chloride as it has a crystal chare of +1
Second elution was a yellow colour expected to be Tris(ethylenediamine)cobalt(III) chloride as it has a crystal charge of +3
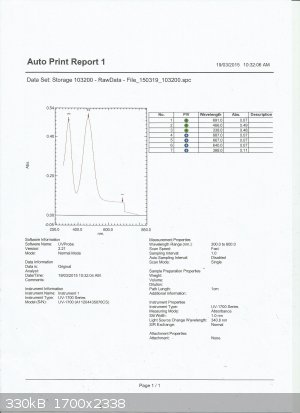
UV-Vis
Atropine, Bicarb, Calcium.
|
|
|
woelen
Super Administrator
        
Posts: 7990
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
What is interesting in this synthesis is the need of acid. In the initial step you add acid to neutralize the ethylene diamine, but I think that this
step is not for neutralizing the ethylene diamine, but to keep a buffer for the base, which is formed when H2O2 is acting as an oxidizer:
H2O2 + 2e --> 2OH(-)
These hydroxide ions interfere and would cause formation of a precipitate of cobaltous or cobaltic hydroxide/oxide and if some ethylene diammonium
chloride is present, then this neutralizes the hydroxide ions. Lateron, when adding 10M HCl, apparently more of this compensation is needed.
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
Nice synthesis. You purified this by chromatography? If so, what solvent system and stationary phase?
|
|
|
Pasrules
Hazard to Self
 
Posts: 78
Registered: 4-1-2015
Location: Yellow Cake Deposit
Member Is Offline
Mood: Lacking an S orbital
|
|
The stationary phase was sephadex cation exchange resin and the mobile phase was 0.5M NaCl which was later replaced by 4M NaCl to elute. The
chromatography was only used to obtain a sample for the UV-Vis.
Atropine, Bicarb, Calcium.
|
|
|
Pasrules
Hazard to Self
 
Posts: 78
Registered: 4-1-2015
Location: Yellow Cake Deposit
Member Is Offline
Mood: Lacking an S orbital
|
|
Just need to make a correction.
The First elution would be dichlorobis(ethylenediamine)cobalt(III) chloride
Atropine, Bicarb, Calcium.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4298
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The green isomer of that can be precipitated from solution with concentrated potassium or sodium nitrate solution. It's surprisingly poorly soluble,
particularly compared to the purple isomer, or the tris en complex.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|